Thermochemistry - PowerPoint PPT Presentation
1 / 14
Title:
Thermochemistry
Description:
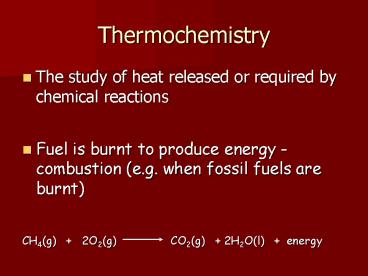
Thermochemistry The study of heat released or required by chemical reactions Fuel is burnt to produce energy - combustion (e.g. when fossil fuels are burnt) – PowerPoint PPT presentation
Number of Views:116
Avg rating:3.0/5.0
Title: Thermochemistry
1
Thermochemistry
- The study of heat released or required by
chemical reactions - Fuel is burnt to produce energy - combustion
(e.g. when fossil fuels are burnt) - CH4(g) 2O2(g) CO2(g)
2H2O(l) energy
2
What is Energy?
3
Chemical Potential Energy Energy stored within
the structural units of chemical
substances Law of Conservation of Energy
the total energy of the universe is constant and
can neither be created nor destroyed it can only
be transformed.
4
Systems Surroundings
In thermodynamics, the world is divided into a
system and its surroundings A system is the part
of the world we want to study (e.g. a reaction
mixture in a flask) The surroundings consist of
everything else outside the system
5
Heat
- The motion of the particles of matter
6
OPEN SYSTEM can exchange both matter and energy
with the surroundings (e.g. open reaction flask,
rocket engine)
CLOSED SYSTEM can exchange only energy with the
surroundings (matter remains fixed) e.g. a sealed
reaction flask
ISOLATED SYSTEM can exchange neither energy nor
matter with its surroundings (e.g. a thermos
flask)
7
Heat Transfer
- Heat flows from an area of high heat to an area
low in heat.
8
Three methods of heat transfer
- Conduction
- Transfer from one substance to another by direct
contact of molecules. Example When you touch a
hot stove. - Convection
- Heat carried from one place to another in a
liquid or gas as molecules move in currents
caused by density differences. Example Warm air
rising. - Radiation
- Heat carried through empty space in the form of
infrared rays. Example When you face the sun
and feel warmth on your face.
9
Temperature
- A measure of the average kinetic energy of
molecules. - The faster the molecules of a substance are
moving, on average, the higher the temperature. - Thermometer - An instrument for measuring
temperature.
10
Temperature Scales
- Celsius -
- Freezing point of water is 0o C.
- Boiling point of water is 100o C.
- Kelvin -
- An "absolute" scale used for kinetic theory
calculations. - Zero on this scale, 0 K, is the lowest possible
temperature. - oC 273 K
- Fahrenheit -
- Freezing point of water is 32 oF.
- Boiling point of water is 212 oF.
- oC (oF - 30) / 2.
11
Absolute Zero
- - The lowest possible temperature.
- The temperature would be 0 K or about -273 oC.
12
Calorie
- The unit of heat.
- The amount of heat needed to raise the
temperature of one gram of water one degree
Celsius. - A "Food Calorie" is 1000 calories, or a
Kilocalorie
13
Specific Heat
- The ability of a substance to absorb heat.
- The specific heat of a substance is the number of
calories needed to raise the temperature of one
gram of the substance one Celsius degree. - The units of Specific Heat are - calories per
gram Celsius degree.
14
EQUATION
- Heat gained or lost (mass) (change in Temp)
(specific heat) - q m x DT x Cp
S.I. unit of energy is the joule (J) Heat and
work ( energy in transit) also measured in
joules 1 kJ (kilojoule) 103 J