1. Mass Spectrometry - PowerPoint PPT Presentation
Title:
1. Mass Spectrometry
Description:
The 1-butene is not symmetrical about the C=C bond, and will this give a small change in dipole during stretching vibration and this the more intense absorption. c. – PowerPoint PPT presentation
Number of Views:54
Avg rating:3.0/5.0
Title: 1. Mass Spectrometry
1
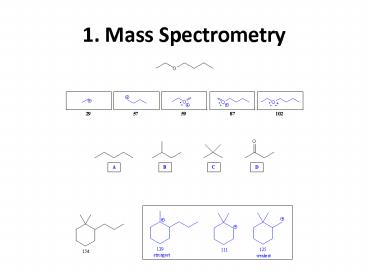
1. Mass Spectrometry
2
1. Mass Spectrometry (contd)
C 6 x 12.0000 H 13 x 1.0078 F 1 x
18.9984 O 1 x 15.9949 120.0950
C 5 x 12.0000 H 12 x 1.0078 O 3 x
15.9949 120.0780
C 8 x 12.0000 H 8 x 1.0078 O 1 x
15.9949 120.0570
C 9 x 12.0000 H 12 x 1.0078
120.0940
3
1. Mass Spectrometry (contd) 2
88 Molecular ion 73 M -15 (loss of
methyl) 70 M -18 (loss of water) 45 M -43
(loss of propyl)
Loss of water indicates the compound is an
alcohol. Alcohols also undergo a-cleavage.
Alcohol that can lose methyl and propyl via a
-cleavage
4
2. Infrared Spectroscopy
- a. Which signal would be more intense in an IR
spectrum, a C-O stretch or a C-N stretch? Why? - Intensity of absorption is correlated to change
in dipole moment with the vibration (stretch).
The C-O bond is more polar and will thus
experience a greater change in dipole moment
during a stretching vibration, giving a more
intense absorption. - b. Which would give a stronger CC stretch in an
IR spectrum, 1-butene or 2-butene? Why? - Intensity of absorption is correlated to change
in dipole moment with the vibration (stretch).
The 2-butene is symmetrical and will not
experience a change in dipole moment during a
stretching vibration. The 1-butene is not
symmetrical about the CC bond, and will this
give a small change in dipole during stretching
vibration and this the more intense absorption. - c. Which would have a higher frequency
absorption, an N-H bond or a P-H bond? Why? - Absorption frequency is correlated to bond
strength and atomic masses. Phosphorus is a
larger atom than nitrogen, giving it a larger
mass and weaker bonding via 3rd shell valence
orbitals compared to nitrogens 2nd shell valence
orbitals. Both of these factors cause the N-H
bond to have a higher frequency absorption than
the P-H bond.
5
2. Infrared Spectroscopy (continued)
Cyclohexene - Presence of CC at 1600 cm-1 -
Absence of CO around 1700 cm-1 - Absence of
O-H around 3300 cm-1 Cyclohexenol -
Presence of O-H at 3300 cm-1 - Absence of CO
around 1700 cm-1 - Absence of CC around 1600
cm-1 Cyclohexanone - Presence of CO at
1700 cm-1 - Absence of CC around 1600 cm-1
- Absence of O-H around 3300 cm-1
6
3. Ultraviolet Spectroscopy
7
3. Ultraviolet Spectroscopy (cont.)
This would have the largest molar absorptivity
(e) and thus the most intense absorption
8
4. NMR Spectroscopy
- If an average carbon requires irradiation at 75
MHz to resonate in a 7.046 T magnetic field, what
frequency will be required to resonate an average
carbon in a 14.092 T magnetic field? - ? ?B0 so doubling the magnetic field
doubles the frequency needed to flip the same
nucleus, 2p therefore 150 MHz is needed. - b. Use the following molecule to answer the
following.
Which carbon is furthest downfield? _____d_____
Which carbon is furthest upfield? _____a____
_ Which carbon resonates at the highest
frequency? _____d_____ Which carbon resonates
at the highest chemical shift? _____d_____ Which
carbon resonates at the lowest chemical
shift? _____a_____ Which carbon is the most
shielded? _____a_____ Which carbon is the
most deshielded? _____d_____
9
4. NMR Spectroscopy (contd)
10
4. NMR Spectroscopy
11
4. NMR Spectroscopy