Bohr Model of Atom - PowerPoint PPT Presentation
Title:
Bohr Model of Atom
Description:
... to the square of the amplitude of the wave Louis deBroglie proposed that the wavelength associated with a matter wave is ... Duality Wave -Particle Duality ... – PowerPoint PPT presentation
Number of Views:182
Avg rating:3.0/5.0
Title: Bohr Model of Atom
1
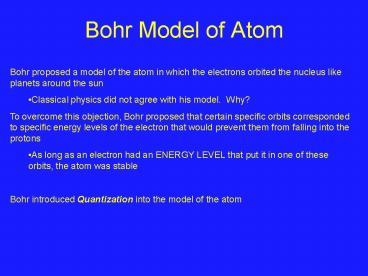
Bohr Model of Atom
- Bohr proposed a model of the atom in which the
electrons orbited the nucleus like planets around
the sun - Classical physics did not agree with his model.
Why? - To overcome this objection, Bohr proposed that
certain specific orbits corresponded to specific
energy levels of the electron that would prevent
them from falling into the protons - As long as an electron had an ENERGY LEVEL that
put it in one of these orbits, the atom was
stable - Bohr introduced Quantization into the model of
the atom
2
Bohr Model of Atom
By blending classical physics (laws of motion)
with quantization, Bohr derived an equation for
the energy possessed by the hydrogen electron in
the nth orbit.
3
Bohr Model of the Atom
- The symbol n in Bohrs equation is the principle
quantum number - It has values of 1, 2, 3, 4,
- It defines the energies of the allowed orbits of
the Hydrogen atom - As n increases, the distance of the electron from
the nucleus increases
4
Atomic Spectra and Bohr
Energy of quantized state - Rhc/n2
- Only orbits where n some positive integer are
permitted. - The energy of an electron in an orbit has a
negative value - An atom with its electrons in the lowest possible
energy level is at GROUND STATE - Atoms with higher energies (ngt1) are in EXCITED
STATES
5
Energy absorption and electron excitation
- If e-s are in quantized energy states, then ?E
of states can have only certain values. This
explains sharp line spectra.
6
Spectra of Excited Atoms
- To move and electron from the n1 to an excited
state, the atom must absorb energy - Depending on the amount of energy the atom
absorbs, an electron may go from n1 to n2, 3, 4
or higher - When the electron goes back to the ground state,
it releases energy corresponding to the
difference in energy levels from final to initial - ?E Efinal - Einitital
- E -Rhc/n2
- ?E -Rhc/nfinal2 - (-Rhc/ninitial2) -Rhc (1/
nfinal2 - 1/ninitial2) - (does the last equation look familiar?)
7
Origin of Line Spectra
Balmer series
8
Atomic Line Spectra and Niels Bohr
- Bohrs theory was a great accomplishment.
- Recd Nobel Prize, 1922
- Problems with theory
- theory only successful for H.
- introduced quantum idea artificially.
- So, we go on to QUANTUM or WAVE MECHANICS
Niels Bohr (1885-1962)
9
Wave-Particle Duality
DeBroglie thought about how light, which is an
electromagnetic wave, could have the property of
a particle, but without mass. He postulated that
all particles should have wavelike
properties This was confirmed by x-ray
diffraction studies
10
Wave-Particle Duality
de Broglie (1924) proposed that all moving
objects have wave properties. For light E
mc2 E h? hc / ? Therefore,
mc h / ? and for particles
(mass)(velocity) h / ?
L. de Broglie (1892-1987)
11
Wave-Particle Duality
- Baseball (115 g) at 100 mph
- ? 1.3 x 10-32 cm
- e- with velocity
- 1.9 x 108 cm/sec
- ? 0.388 nm
- The mass times the velocity of the ball is very
large, so the wavelength is very small for the
baseball - The deBroglie equation is only useful for
particles of very small mass
12
1.6 The Uncertainty Principle
- Wave-Particle Duality
- Represented a Paradigm shift for our
understanding of reality! - In the Particle Model of electromagnetic
radiation, the intensity of the radiation is
proportional to the of photons present _at_ each
instant - In the Wave Model of electromagnetic radiation,
the intensity is proportional to the square of
the amplitude of the wave - Louis deBroglie proposed that the wavelength
associated with a matter wave is inversely
proportional to the particles mass
13
deBroglie Relationship
- In Classical Mechanics, we caqn easily determine
the trajectory of a particle - A trajectory is the path on which the location
and linear momentum of the particle can be known
exactly at each instant - With Wave-Particle Duality
- We cannot specify the precise location of a
particle acting as a wave - We may know its linear momentum and its
wavelength with a high degree of precision - But the location of a wave? Not so much.
14
The Uncertainty Principle
- We may know the limits of where an electron will
be around the nucleus (defined by the energy
level), but where is the electron exactly? - Even if we knew that, we could not say where it
would be the next moment - The Complementarity of location and momentum
- If we know one, we cannot know the other exactly.
15
Heisenbergs Uncertainty Principle
- If the location of a particle is known to within
an uncertainty ?x, then the linear momentum, p,
parallel to the x-axis can be simultaneously
known to within an uncertainty, ?p, where - h/2? hbar
- 1.055x10-34 Js
- The product of the uncertainties cannot be less
than a certain constant value. If the ?x
(positional uncertainty) is very small, then the
uncertainty in linear momentum, ?p, must be very
large (and vice versa)
?
16
Wavefunctions and Energy Levels
- Erwin SchrÖdinger introduced the central concept
of quantum theory in 1927 - He replaced the particles trajectory with a
wavefunction - A wavefunction is a mathematical function whose
values vary with position - Max Born interpreted the mathematics as follows
- The probability of finding the particle in a
region is proportional to the value of the
probability density (?2) in that region.
17
The Born Interpretation
- ?2 is a probabilty density
- The probability that the particle will be found
in a small region multiplied by the volume of the
region. - In problems, you will be given the value of ?2
and the value of the volume around the region.
?
18
The Born Interpretation
- Whenever ?2 is large, the particle has a high
probability density (and, therefore a HIGH
probability of existing in the region chosen) - Whenever ?2 is small, the particle has a low
probability density (and, therefore a LOW
probability of existing in the region chosen) - Whenever ?, and therefore, ?2, is equal to zero,
the particle has ZERO probability density. - This happens at nodes.
19
SchrÖdingers Equation
- Allows us to calculate the wavefunction for any
particle - The SchrÖdinger equation calculates both
wavefunction AND energy
Potential Energy (for charged particles it is the
electrical potential Energy)
Curvature of the wavefunction
20
Particle in a Box
- Working with SchrÖdingers equation
- Assume we have a single particle of mass m stuck
in a one-dimensional box with a distance L
between the walls. - Only certain wavelengths can exist within the
box. - Same as a stretched string can only support
certain wavelengths
21
Standing Waves
22
Particle in a Box
- The wavefunctions for the particle are identical
to the displacements of a stretched string as it
vibrates.
where n1,2,3,
- n is the quantum number
- It defines a state
?
23
Particle In a Box
- Now we know that the allowable energies are
Where n1,2,3,
- This tells us that
- The energy levels for heavier particles are less
than those of lighter particles. - As the length b/w the walls decreases, the
distance b/w energy levels increases. - The energy levels are Quantized.
?
24
Particle in a BoxEnergy Levels and Mass
- As the mass of the particle increases, the
separation between energy levels decreases - This is why no one observed quantization until
Bohr looked at the smallest possible atom,
hydrogen
m1 lt m2
25
Zero Point Energy
- A particle in a container CANNOT have zero energy
- A container could be an atom, a box, etc.
- The lowest energy (when n1) is
Zero Point Energy
- This is in agreement with the Uncertainty
Principle - ?p and ?x are never zero, therefore the particle
is always moving
26
Wavefunctions and Probability Densities
- Examine the 2 lowest energy functions n1 and n2
- We see from the shading that when n1, ?2 is at a
maximum _at_ the center of the box. - When n2, we see that ?2 is at a maximum on
either side of the center of the box
27
Wavefunction Summary
- The probability density for a particle at a
location is proportional to the square of the
wavefunction at the point - The wavefunction is found by solving the
SchrÖdinger equation for the particle. - When the equation is solved to the appropriate
boundary conditons, it is found that the particle
can only posses certain discrete energies.