Chapter 12. Light scattering (determination of MW without calibration) - PowerPoint PPT Presentation
1 / 44
Title:
Chapter 12. Light scattering (determination of MW without calibration)
Description:
Chapter 12. Light scattering (determination of MW without calibration) Electromagnetic radiation : – PowerPoint PPT presentation
Number of Views:908
Avg rating:3.0/5.0
Title: Chapter 12. Light scattering (determination of MW without calibration)
1
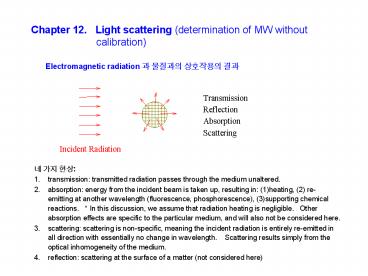
Chapter 12. Light scattering (determination of
MW without calibration)
Electromagnetic radiation ? ???? ????? ??
- ? ?? ??
- transmission transmitted radiation passes
through the medium unaltered. - absorption energy from the incident beam is
taken up, resulting in (1)heating, (2)
re-emitting at another wavelength (fluorescence,
phosphorescence), (3)supporting chemical
reactions. In this discussion, we assume that
radiation heating is negligible. Other
absorption effects are specific to the particular
medium, and will also not be considered here. - scattering scattering is non-specific, meaning
the incident radiation is entirely re-emitted in
all direction with essentially no change in
wavelength. Scattering results simply from the
optical inhomogeneity of the medium. - reflection scattering at the surface of a matter
(not considered here)
2
- Now we focus on the light scattering.
- Application of Light Scattering for Analysis
- Classical Light Scattering (CLS) or Static Light
Scattering (SLS) - Dynamic Light Scattering (DLS, QELS, PCS)
- CLS
- ?? Scattering center small volumes of material
that scatters light. ? individual molecule in
a gas. - Consequences of the interaction of the beam with
the scattering center depends, among other
things, on the ratio of the size of the
scattering center to the incident wavelength
(?o). Our primary interest is the case where
the radius of the scattering center, a, is much
smaller than the wavelength of the incident light
(a lt 0.05?o, less than 5 of ?o). This
condition is satisfied by dissolved polymer coils
of moderate molar mass radiated by VISIBLE light.
When the oscillating electric field of the
incident beam interacts with the scattering
center, it induces a synchronous oscillating
dipole, which re-emits the electromagnetic energy
in all directions. Scattering under these
circumstances is called Rayleigh scattering.
The light which is not scattered is transmitted
, where Is and It are the
intensity of the scattered and transmitted light,
respectively.
3
- Oscillating electric field of incident beam
interacts with scattering center, induces a
synchronous oscillating dipole, which re-emits
electromagnetic energy in all directions.
Rayleigh scattering? ?? ???? ??? ?? ??? ?? ???
(1cos2?)? ????, scattering center? observer???
??(r)? ??? ???.
- 1944, Debye
- Rearrange
Constant, K
?o ?????, dn/dc refractive index
increment no ??? refractive index, p???,
c????g/mL
4
I? is inversely proportional to ?o. Shorter
wavelength scatters more than longer
wavelength Assume system is dilute, the net
signal at the point of observation is sum of all
scattering intensities from individual scatterer
- no multiple scattering (scattered light from
one center strike another center causing
re-scattering, etc.).
- Two ways to access the light scattering
information experimentally - Turbidimeter (or spectrophotometer)
- Light scattering
5
1. Turbidimeter experiment (Transmitted light
intensity, It is measured)
Solution is dilute, so higher order concentration
terms can be ignored.
6
Procedure Measure t at various conc. ? Plot Hc/T
vs. c (straight line) ? Determine M from
intercept, 2nd virial coeff., B from slope
7
2. Light Scattering experiment (measure I? at
certain ? and r)
?6? ?4? ??
??? ??? ? 5 (?/20) ??? ??? ??? Rayleigh
limit
8
?-condition?? ???0.
9
lt??gt For polydisperse sample, Turbidity (?? light
scattering) is contributed by molecules of
different MW. Define ti ??? Mi? ?? ???? ??
turbidity ?
??? turbidity? light scattering???? ?? ????
weight-average MW??.
10
Rayleigh-Gans-Debye (RGD scattering) when the
scattering centers are larger than Rayleigh limit
Different part of more extended domain (B)
produce scattered light which interferes with
that produced by other part (A) - constructive or
destructive
11
Distribution is symmetrical for small particles
(lt?/20). For larger particles, intensity is
reduced at all angles except zero.
Contributions from two scattering centers can be
summed to give the net scattering intensity.
The result is a net reduction of the scattered
intensity
P? "shape factor" or "form factor"
Always P? lt 1, function of size and shape of
scattering volume. Now we start seeing the
angle dependence of the scattered light !
12
- p(?) decreases with ?.
- p(?) decreases more for higher MW.
13
Effect of MW and Chain Conformation on P?, and on
measured MW at 90o.
Conformation MW (g/mol) RG (nm) P(90o) MW(90o)
Random coil
Polystyrene 51K 8 0.98 51K
Polystyrene( ? condition) 420K 19 0.95 400K
PMMA 680K 36 0.70 480K
Polyisoprene(70 cis) 940K 48 0.56 530K
Spherical
Bovine serum albumin 66K 3 1.00 66K
Bushy stunt virus 10700K 12 0.98 10500K
Rod shaped
Poly- -benzyl-L-glutamate 130K 26 0.91 118K
Myosin 493K 47 0.74 365K
DNA 4000K 117 0.35 1400K
14
Final Rayleigh equation for random coil polymer
? ?? ?? ??
Case 1 ??0
Plot Kc/R? vs. c y-??1/M, ???2A2
Case 2 c?0
Plot Kc/R? vs. sin2(?/2) y-??1/M, ???
(16p2/3M?2) rg2
Three information!
15
??? ?? ?? (1) ??? ??? ???? R???. (2) Kc/R? vs.
c, Kc/R? vs. sin2(? /2) plot ??. (3) ? 0 ? c
0 ? extrapolate.
Kc/R? vs. sin2(? /2)
Kc/R? vs. c
Zimm plot
??? ? ?? ???. ? ? extrapolated points
16
Cases
1. Small polymers ????? ??. (Horizontal line)
Zimm plot for PMMA in butanone ?o546 nm, 25?, no
1.348, dn/dc 0.112 cm3/g
- ?? ???? ??? ???. - Mw ? A2 ?? ?? - ???? ?? ???.
2. Small polymers in ?-solvent ?? ? ?? ??? ??.
Zimm plot of poly(2-hydroxyethyl methacrylate) in
isopropanol ?o436 nm, 25?, no 1.391, dn/dc
0.125 cm3/g
?-solvent A20? ?? ??, ???-???, ???-????? ?????
???? ??, ????? ?? ??.
- Calculated values Mw 66,000 g/mol
- A2 0 mol
cm3/g2 - - Kc/R? at small angles fall mostly below the
horizontal line plotted through the points from
medium and large angles.
17
3. Larger polymers in good solvent ?? ? ??? ??.
Zimm plot of polystyrene in toluene ?o546 nm,
25?, no 1.498, dn/dc 0.110 cm3/g
- ??? ? 2x105 ??? ??, Kc/R? ? ?? ??? (A2??)?
???. - Athermal Condition - No effect of
temperature on polymer structure
4. Polymers in poor solvent A2 ? ??? ? (? ???
? ? ??. ? ?? ?? ?? ??)
Zimm plot of polybutadiene in dioxane ?o546 nm,
25?, no 1.422, dn/dc 0.110 cm3/g
- - ?????? ??? ?? (nonlinear).
- - ?? microgel, ??, aggregate? ?? ? ?? ??.
- Curve-fitting? ??? ??.
- ???? ??? ???? ??.
- ??? ??? good solvent???
- ???? ??? ? ??.
18
Stand-alone vs. On-line MALS
- ltStand-alone modegt
- Stand-alone mode LS instrument is used itself.
- Zimm plot ? ?? M, A2, R?? ??
- ltOn-line modegt
- LS instrument is used as a detector for a
separator. - c0 ?? ??.
- ? slice? ?? Kc/R? vs. sin2(?/2) ???? ??,
y-?????? ??? (M), ???????? rg? ??. y-??1/M,
????? (16p2/3M?2) rg2 - ? slice? monodisperse??? ???? ?????? ????? ??.
??? ?? ???? ??? (?????? ? ?????? ???).
- Average Molecular Weights
- No-average Mn(Sci)/(S(ci/Mi))
- Wt-average MwS(ci Mi)/ S(ci)
- Z-average Mz S(ci Mi2)/S(ci/Mi)
- Average Sizes (mean square radii)
- No-average ltrg2gtn S(ci/Mi)ltrg2gti/S(ci/Mi)
- Wt-average ltrg2gtw S(ciltrg2gti)/Sci
- Z-average ltrg2gtzS(ciMiltrg2gti)/S(ciMi)
19
Light scattering instruments MALLS (Multi Angle
Laser Light Scattering) I? is measured at 15
angles (1) Stand-alone mode Measure scattered
light at different angles for different
concentrations ? Make a Zimm plot ? Determine M,
B, Rg
Assuming each slice is narrow distribution, Mw ?
Mi Average M can be calculated. It is therefore
very important to have a good resolution.
20
21
Angular Dependence of Kc / R?(?? high molecular
weight DNA)
22
Effect of Particles/Gels on Light Scattering
Measurement Note the delicacy of extrapolation to
zero angle from larger distances.
23
- DALLS (Dual Angle) I?? is measured at 15o and
90o - LALLS (Low Angle) I? is measured at one low
angle (assume ?? 0) - Static mode measure LS at a few c ? Plot Kc/R?
vs. c ? Determine M and B from intercept and
slope. - On-line mode determine Kc/R? for each slice (
calculate M). Considering each slice is
narrow distribution, let Mw ( Mi, from which
average MW's can be calculated (as learned in
chapter 1). It is therefore again very
important to have a good resolution.
- RALLS (Right Angle)
- I? is measured at 90o.
- Simple design
- Higher S/N ratio, Application is limited to
cases where P? is close to 1 (e.g., less than
200K of linear random polymer) - RALLS combined with differential viscometer
(commercially available from Viscotek, "TRISEC")
24
ltTRISEC ?? ??gt Assume P? 1 and A2 0.
Determine Mest.
? is determined by differential viscometer, and
M determined in step 2.
Calculate new MW by
Go to step 2. Repeat until Mest does not change.
25
ltLight scattering experiment? ??? ???gt
?? K? B? ??? ?? parameter? ?? ?? ??. ???
??? ?? ? ?? ??? ??.
- n ??? refractive index
- dn/dc Specific refractive index increment
- B 2nd virial coefficient (Static mode??? B? ???
?? ??? ? ?? ??? Static mode? ??).
26
1. ??? Refractive Index ?? ?? ??? ?? RI ??? ???
??.
?? ??? ??? (R?? ???? ?)
Solvent RI R? x 106 cm-1
Carbon disulfide 1.6207 57.5
a-chloronaphthalene (140 oC) 1.5323 52.8
1,2,4-Trichlorobenzene (135 oC) 1.502 35.7
Chlorobenzene 1.5187 18.6
o-Xylene (35 oC) 1.50 15.5
Toluene 1.49 14.1
Benzene 1.50 12.6
Chloroform 1.444 6.9
Methylene chloride 1.4223 6.3
Carbon tetrachloride 1.46 6.2
Dimethyl formamide 1.43 (589 nm) 5.6
Cyclohexane 1.425 5.1
Cyclohexanone 1.4466 4.7
Methyl ethyl ketone 1.38 4.5
Ethyle acetate 1.37 4.4
THF 1.41 4.4
Acetone 1.36 4.3
Dimethyl sulfoxide 1.478 (589 nm) 4.1
Methanol 1.33 2.9
Water 1.33 1.2
- Except where otherwise noted, all measurements
made at ? 632.8 nm and T23 oC. RI at 632.8 nm
calculated by extrapolation from values measured
at other wavelengths. - Extrapolation? ?? reference Johnson, B. L.
Smith, J. "Light Scattering from Polymer
solutions" Huglin, M. B. ed., Academic press, New
York, 1972, pp 27
27
2. Specific refractive Index, dn/dc
- ???? ?? ? ?? (Polymer Handbook, Huglin, ed.,
Light Scattering from Polymer Solutions, Academic
Press, 1972) - ???? ?? ? ?? ?? ??? ?? ??
- Conventional method
- DRI? ??
- ? ?? ?? ???? (n2-n1)? ?? (recommended conc. 2,
3, 4, 5 x 10-3 g/mL) ? (n2-n1)/c2 vs. vs. c2?
plot ? zero concentration?? extrapolate ? dn/dc?
intercept? ? ? ???.
28
For concentration ranges generally used, the
refractive index difference, n2-n1, is a linear
function of concentration. In other words,
(n2-n1)/c2 is constant. ? (n2-n1)/c2 vs. c2 ????
???0.
This means that (n2-n1) needs to be measured for
only one or two different concentrations. If
(n2-n1)/c2 shows no significant dependence on c,
then dn/dc can be obtained by averaging
(n2-n1)/c2 values
29
- SEC/RI? ??
- ?? ?? ?? ??
?? dn/dc? ?? ????? ???? kR? ??
- ???? ??? ?? ?? ? ?? ?? estimate? ? ?? ??.
- extrapolate to desired wavelength
?? 2) polymer? ??? refractive index? ??
estimate
???? n2? polymer? partial specific volume
mL/g??. ?? n2 ? 1.
- lt????gt
- dn/dc ? ??? ????? light scattering ??? ?? ??? ???
??? ?? ???? ???? ??. - Dn/dc ? ??? ????? ???? ??? ??. Dn/dc ? ???? ??.
- ??? dn/dc ?? ??. ???? ???? ?? ??? ??.
30
3. Virial Coefficient, B or A2
- ???? ?? ? ?? (? Polymer Handbook). ???? ?? ? ??
?? ??? ?? ?? (stand-alone Light scattering) - 2nd Virial Coefficient? Solute-Solvent
interaction? ??. - Polymer-solvent interaction, good solvent (the
higher, the better solvent). - 0 Unperturbed system
- - Polymer-polymer interaction, poor solvent.
- A2? ???? ?? A2 b M-a ? log A2 vs. log M?
??. ?? ???? ??, ? ???? ???. - dn/dc? A2? ???? ?? ???? S. Lee, O.-S. Kwon,
"Determination of Molecular Weight and Size of
Ultrahigh Molecular Weight PMMA Using Thermal
Field-Flow Fractionation/Light Scattering" In
Chromatographic Characterization of Polymers.
Hyphenated and Multidimensional Techniques,
Provder, T., Barth, H. G., and Urban, M. W. Ed.
Advances in Chemistry Ser. No. 247 ACS
Washington, D. C., 1995 pp93.
31
- Light scattering ??? ? ? ?? ?? ? ?? (concerns)
- ??? dn/dc, RI constant, A2? ??.
- As dn/dc increases, calculated MW decrease,
calculated mass decrease, and no effect on
calculated RG. - As RI constant increases, calculated MW
decreases, calculated mass increases, and no
effect on RG . - As A2 increases, calculated MW increases, no
effect on calculated mass, RG slightly increases. - Refractive Index Detector Calibration ? ????? ?
?? - RI Calibration constant inversely proportional
to the detector sensitivity. - Sensitivity of most RI detector is
solvent-dependent. - A calibration constant measured in a solvent may
not be accurate for other solvents. It is
recommended to use a solvent that will be used
most often (e.g., THF or toluene). - For RI calibration, only the RI signal is used.
Light scattering instrument calibration is not
needed. - Concentration of standards should be such that
the output of RI detector varies between about
0.1 - 1.0 V and should correspond to normal peak
heights of samples (For a Waters 410 RI at
sensitivity setting of 64, this corresponds
roughly to concentrations of 0.1 - 1.0 mg/mL. RI
output can be usually monitored by light
scattering instrument (e.g., channel 26 of DAWN). - Use NaCl in water as a standard for aqueous
system. - The RI calibration constant will change if you
change the sensitivity setting of the detector
So it is important to use the same sensitivity
setting of RI detector as that used when the
detector was calibrated.
32
- RI calibration preparation One Manual injector
with at least 2 mL loop, Five or more known
concentrations (0.1 - 1 mg/mL) of about 200 K
polystyrene in THF. - RI calibration Procedure
- Remove columns. Place manual injector with loop.
- Pump THF through a RI detector at normal flow
rate (about 1 mL/min). Purge both reference and
sample cells of detector until baseline becomes
flat stable. - Stop purging and wait till baseline becomes
stable. - Set up the light scattering data collection
software (enter filename, dn/dc, etc.) Enter 1 x
10-4 for RI constant (light scattering instrument
usually requires the RI constants to be entered).
Set about 60 mL for Duration of Collect . - Begin collecting data with ASTRA.
- Inject pure solvent first followed by stds from
low to high conc, and finish with pure solvent.
- Repeat the measurements if you want.
- Data Analysis (1)set baseline using signals from
pure solvent at the beginning and the end
(2)calculate each concentration as a separate
peak by marking exactly 1 mL as peak width (or 30
slices at 1 mL/min, 2 seconds of collection
interval).(3)calculate the mass of the peak
(4)plot the injected mass (y-axis) vs. calculated
mass (x-axis) (5)do linear regression on data by
forcing the intercept be zero (6)calculate RI
constant using RI constant slope x 1x10-4
33
- Chemical heterogeneity within each slice leads to
non-defined dn/dc ? Quantitation of chemical
heterogeneous samples is very difficult. - Limited sensitivity to low MW components.
Mn(exp)gtMn(true). The same concern with
differential viscometer experiments. - Limited Sensitivity of Light Scattering and RI
Detector
- g' values may be in error if each peak slice
contains both linear and branched polymer or
different types of long-chain branching g' will
be overestimated. - Quality of data is highly affected by the
presence of particles. - Lower limit of RG with MALS? ? 10 nm (about 100K
MW) - Inter-detector volume must be known accurately.
34
Comparison of online LS vs. viscometer
LS Viscometer
MWD Absolute Relative
need precise n and dn/dc Universal calibration must be valid or need M-H coefficient
independent of separation mechanism Independent of separation mechanism if M-H coefficients are used. Dependent on separation mechanism if universal calibration is used.
? distribution indirect from universal calibration direct, independent of separation mechanism
RG direct from MALS (limited to gt10 nm) indirect from universal cal. and Flory-Fox eqn. applicable to linear molecules only
Chain conformation MALLS RG vs. M plot ? vs. M plot (M-H coefficients can be obtained) RG vs. M plot.
Branching g obtained directly from MALS, indirectly from LALLS universal calibration g' obtained directly
heterogeneous samples limited because of dn/dc uncertainty directly applicable with univ. calib., but the change in dn/dc will affect DRI responses
Lower MW detectability 2K. depends on dn/dc and polydispersity as low as 300-400 has been reported
Response to particle contamination LALLS highly sensitive, MALLS less sensitive Insensitive
35
Information Content
Primary Secondary
LALLS M
MALLS M RG
PCS D Rh, M
Viscometer ? M, RG
Primary information high precision and accuracy,
insensitive to SEC variables, requires no SEC
column calibration.
36
ltSEC-VISC-LS instrumentgt
- Features
- MWD measured by LS
- IVD measured by Viscometer
37
- Both Viscometer and LS are insensitive to
experimental conditions and separation mechanism - No band broadening corrections are needed for Mw,
? , a, k, and g - Precise and accurate calculation of hydrodynamic
radius distribution, M-H constants, and Branching
distribution
38
- Dynamic light scattering (DLS, QELS, PCS)
- Classical light scattering "time-averaged
scattering intensity"? ?? ???? ??? ? scattering
center??? ?? ?? ?? ??? ? (algebraic summation).
- ??? algebraic summation? ??? ? ???? random??
array????, ?? phase relationship? scattering
volume dimension? ??? ?? ?? ??? ?????? ??
interference effect ?? average-out?? ??? ????
???. - Scattering volume dimension? ?? ???, ???? ??? ?
scattering center? ?? ?? ?? ?? ?? ??? interfere
(constructive or destructive) ???? ?? ???? ???
???? ???? ??? ?? ????. - ? ???? Brownian motion (diffusion) ? ?? ?? ?????
???? ???? ?? ?? ?? ????. ??? ???? ???? ??? ???
?? fluctuate??. - Fluctuate?? ??? ???? diffusion rate? ??
(diffusion rate? ???? ??? fluctuate). - nanometer ?? micron??? ??? ??? ???? ?? viscosity?
??? viscosity? ??? media? disperse?? ?? ?, ????
??? ?? ?? (fluctuation)? microsecond ??
millisecond??.
39
- A vertically polarized laser beam is scattered
from a colloidal dispersion. The
photomultiplier detects single photons scattered
in the horizontal plane at an angle ? from the
incident beam, and the technique is referred to
as "photon correlation spectroscopy (PCS) - Because the particles are undergoing Brownian
motion, there is a time fluctuation of the
scattered light intensity, as seen by the
detector. The particles are continually
diffusing about their equilibrium positions.
Analyzing the intensity fluctuations with a
correlator yields the effect diffusivity of the
particles. - Measured intensity, I vector sum of scattering
from each particle - Brownian motion motion caused by thermal
agitation, that is, the random collision of
particles in solution with solvent molecules.
These collisions result in random movement that
causes suspended particles to diffuse through the
solution. For a solution of given viscosity, ?,
at a constant temperature, T, the rate of
diffusion (diffusion coefficient) D is given by
the Stokes-Einstein equation, D(kT)/(6p?d),
where k Boltzman's constant, d equivalent
spherical hydrodynamic diameter. ??? diffusion
coefficient (D)? ?????? ?? ?? (?? ???)? ??? ? ??. - DLS??? ? ??? ??? ?? ?? ???? ??? ?? ??(t time
interval)?? ???? ??? ????. ???? ??? ???? ???
??? t? ?? ?, I(0)? I(t)? ??. ?? ?? ?? interval?
?? ???? I(0)? I(t)? ??? ? intensity product,
I(0)I(t)? ???? ltI2(0)gt, ? average of the square
of the instantaneous intensity ? ???? - ?? "I(0)?
I(t)? correlate????"?? ??. ???? ??? ???? ???
??? t? ? ?, I(0)? I(t)? ??? ??? ?? ??? - "I(0)?
I(t)? correlate???? ??" ?? "I(0)? I(t)?
un-correlate ????" ?? ??. ???? intensity
product, I(0)I(t)? ???? ??? ltI2gt, ? square of the
long-time averaged intensity? ??. ???? ??? ????
??? ??? t? ??? ??? ?? ?, "I(0)? I(t)? ?????
correlate????".
40
- Measured intensity, I vector sum of scattering
from each particle - Measure I at various time interval, ?,
- I(0) I(t) for short t ? correlated,
correlation decreases as ? increases. - I(0)? I(t)? ?????? Correlation? ??? ??? ? ??.
correlation? ??? ???? ?? average of the intensity
product, G(t)? ????. - ?? G(t)Anto correlation function
ltI(t)I(tt)gt average of the intensity product.
- ?? ???? t ? ???? ?? G(t)? ??.
- G(t) is high for high correlation, and is low for
low correlation. - High correlation means that particles have not
diffused very far during t. Thus G(t)
remaining high for a long time interval indicates
large, slowly moving particles. - The time scale of fluctuation is called "decay
time - Decay time is directly related with the particle
size. The inverse of decay time is the decay
constant, ?. - Usefulness of G(t) directly relatable to the
particle diffusivity - For monodisperse samples,
41
?? ?? ??? ?? ??? interval?? autocorrelation
function, G(t)? ???
G(t) vs. t? ???? ??? Exponential function? ????
G(t)? fit??.
Rh? ??, ??? ?? ???? Measure I(t) at various ? ?
G(t) ?
?? DLS ? ??? ???? diffusion? ?? ??? ?? ?? ??
dispersion ( ? 0.03)? ??? ???. ? volume
fraction of suspended spheres.
, where N Avogadro's no., M MW, Vh
hydrodynamic vol.). Infinite dilution D?? ??
???? ?? ? 0.005? ?? ??? ??.
42
???? f(a) distribution function, I(a,?)
scattering intensity function for RGD spheres.
PC? ??, normal?? log-normal distribution
function? G(t)? fit??.
?? Narrow, mono-modal distribution ??? ??,
"method of cumulant"? ??, ??? ?? ??? ? ??.
- ???? an nth moment of f(a).
- We see that DLS yields a somewhat unusual
average radius (the inverse "z-average", and one
which is quite highly sensitive to the presence
of outsized particles. - DLS uses a single exponential decay function, and
thus it does not give information on sample
polydispersity.
43
- ??
- RI values of medium and sample are needed for DLS
experiments. - RI 1.333 for water, and 1.5 - 1.55 for typical
polymers and proteins. - RI of sample is needed only when the intensity
weight needs to be converted to the volume weight
(e.g., for samples having broad distributions).
- Theory to convert the intensity to the volume
is only for solid particles. So the conversion
will not be accurate for samples such as
liposomes which are hollow inside. - For samples such as liposome, a value between 1.5
- 1.55 can be used as it is typical values for
polymers and proteins. - For samples having narrow distributions, only the
unimodal analysis is performed, and thus there is
no need to convert the intensity to the volume
. - RI value will not make any difference in the
average size data because only the RI of medium
is need for unimodal analysis.
DLS summary
- D depends on MW and conformation
- Diffusion coefficient distribution can be
obtained - D is independent on chemical composition. ?D can
be obtained without knowing chemical composition. - Concentration is not needed to determine D
- Input parameters (T, n, ? ) are easily measured.
- Concerns sensitivity, interference from
particulates, inconsistency, not very useful
for polydispersed or multi-modal distributions.
44
lt??gt Particle Size Conversion Table
Mesh size Approximate ?µ size
4 4760
6 3360
8 2380
12 1680
16 1190
20 840
30 590
40 420
50 297
60 250
70 210
80 177
100 149
140 105
200 74
230 62
270 53
325 44
400 37
625 20
1250 10
2500 5