Raman - PowerPoint PPT Presentation
Title:
Raman
Description:
Raman Spectroscopy Laser 4880 Raman Spectroscopy Selection Rules Infrared: Intensity of a peak is related to the change in the dipole moment associated in going ... – PowerPoint PPT presentation
Number of Views:335
Avg rating:3.0/5.0
Title: Raman
1
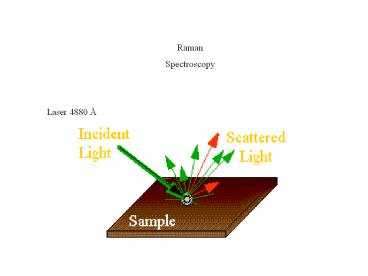
Raman Spectroscopy
Laser 4880 Å
2
Raman Spectroscopy
3
(No Transcript)
4
Selection Rules Infrared Intensity of a peak is
related to the change in the dipole moment
associated in going from the ground state to an
excited state the principle vibrational quantum
number changes by 1 Raman Intensity of a peak
is related to the polarizability of the stretch.
Non-polar bonds are usually more easily polarized
than polar bonds.
5
Comparison of IR and Raman Spectroscopy 1. Bands
that are intense in the IR are usually weak in
the Raman. The spectral interference associated
with hydrogen bonding is greatly reduced. Similar
reduction in interference can also be obtain by
examining gas phase spectra. Water is a useful
solvent in Raman whereas water is a poor solvent
for IR studies. The optics in Raman are made from
glass or quartz instead of salts (NaCl, KBr, CsI).
6
Liquid film
NH2CH2CH2OH
7
KBr
8
Comparison of IR and Raman Spectroscopy 1. Bands
that are intense in the IR are usually weak in
the Raman. The spectral interference associated
with hydrogen bonding is greatly reduced. Similar
reduction in interference can also be obtain by
examining gas phase spectra. Water is a useful
solvent in Raman whereas water is a poor solvent
for IR studies. The optics in Raman are made from
glass or quartz instead of salts (NaCl, KBr,
CsI). 2. Molecules with a center of symmetry have
no coincident IR and Raman bands. Thus a
comparison of the two spectra can provide
structural information. 3. Raman spectra are
generally simpler that IR spectra. Overtones and
combination bands frequent in IR are less common.
9
(No Transcript)
10
KBr
powder
11
KBr
powder
12
Comparison of IR and Raman Spectroscopy 1. Bands
that are intense in the IR are usually weak in
the Raman. The spectral interference associated
with hydrogen bonding is greatly reduced. Similar
reduction in interference can also be obtain by
examining gas phase spectra. Water is a useful
solvent in Raman whereas water is a poor solvent
for IR studies. The optics in Raman are made from
glass or quartz instead of salts (NaCl, KBr,
CsI). 2. Molecules with a center of symmetry have
no coincident IR and Raman bands. Thus a
comparison of the two spectra can provide
structural information. 3. Raman spectra are
generally simpler that IR spectra. Overtones and
combination bands frequent in IR are less common.
13
Comparison of IR and Raman Spectroscopy 2. Molecul
es with a center of symmetry have no coincident
IR and Raman bands. Thus a comparison of the two
spectra can provide structural information. 3. Ram
an spectra are generally simpler that IR spectra.
Overtones and combination bands frequent in IR
are less common. 4. The entire IR range can be
covered by Raman spectroscopy since a laser,
usually in the visible region is used and the
spectrum is obtained by looking at the frequency
differences from the incident frequency. In IR,
different optics and beam-splitters are needed to
cover the entire useful range from the near IR to
the far IR.
14
Comparison of IR and Raman Spectroscopy 5. IR
spectrometers are less expensive and more
sensitive instruments. Intensity measurement in
Raman are very sensitive to laser power, and cell
geometry, and are less reproducible than IR
spectra. 6. A small fraction of the incident
photons in Raman are scattered, (e. g. 10-8).
Broadband fluorescence can obscure the Raman
signals. 7. As a result of the simplification in
the spectra, Raman spectroscopy provides less
structural information.
15
Liquid film
liquid
16
(No Transcript)
17
(No Transcript)
18
D serine
19
DL
20
(No Transcript)
21
(No Transcript)





























![Dr C V Raman University - [CVRU], Bilaspur PowerPoint PPT Presentation](https://s3.amazonaws.com/images.powershow.com/9656044.th0.jpg?_=20210903018)
