Chapter 3 Biochemistry - PowerPoint PPT Presentation
1 / 83
Title:
Chapter 3 Biochemistry
Description:
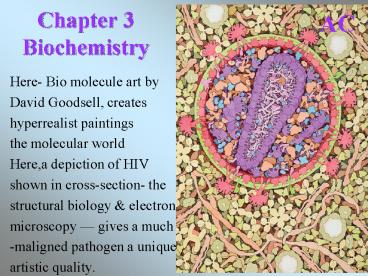
AC Chapter 3 Biochemistry Here- Bio molecule art by David Goodsell, creates hyperrealist paintings the molecular world Here,a depiction of HIV shown in cross-section- the – PowerPoint PPT presentation
Number of Views:215
Avg rating:3.0/5.0
Title: Chapter 3 Biochemistry
1
Chapter 3 Biochemistry
AC
- Here- Bio molecule art by
- David Goodsell, creates
- hyperrealist paintings
- the molecular world
- Here,a depiction of HIV
- shown in cross-section- the
- structural biology electron
- microscopy gives a much
- -maligned pathogen a unique
- artistic quality.
2
Examples of bio molecules Hemoglobin
- the oxygen-carrying molecule of erythrocytes,
formed by developing erythrocytes in the bone
marrow - a protein made up
- of 4 polypeptide
- chains that contain
- 141 - 146 amino
- acids each.
3
Examples of biological molecules
- The Helicase enzyme unzips the DNA molecule, to
expose the nitrogenous bases so we can read the
genetic code do everything that is in the
instructions for life.
4
Inside leaves, solar energy is transferred to the
chemical bonds in biological molecules like
GLUCOSE food.
5
Examples Biological molecules
- Hormones are chemical messengers that regulate
imperative bodily functions in living organisms. - For example pheromones are hormones used by ants
other insects as a - communication system to
- send messages among one
- another or to attract the
- opposite sex.
http//www.reciprocalnet.org/edumodules/commonmole
cules/biochemical/index.html
6
Examples biological molecules
- Other examples of biomolecules are poisons found
in animals. Batrachotoxin, a poison at the skin
of the golden poison frog - Phyllobates terribilis is considered
- to be one of the most deadly poisons to humans,
however it is harmless to predator, - the snake Liophis epinephelus .
bio.davidson.edu
7
- Before you can study any of these complex
systems, you must understand the molecules that
are behind all the wonder..
8
Remember!
CHNOPS the 6 most common elements in living
things
- Biological molecules, are built by joining atoms
through covalent bonds. - Although more than 25 types of elements can be
found in biomolecules, 6 elements are most
common. These are called the CHNOPS
9
All compounds can be classified into 2 broad
categories
- Organic compounds
- made primarily of carbon atoms.
- Most matter in living organisms that is not water
is made of organic compounds. - Inorganic compounds
- Compounds that, with a few exceptions, do not
contain carbon atoms. (an exception is CO2- not
organic!) water is ex. of an inorganic compound.
10
What do people think organic means?
- A survey showed most people think organic
refers to organic foods (limited use of synthetic
materials during growth production), and only a
few science majors said carbon compounds. - Organic chemistry is - the study of the
structure, properties, and reactions of carbon
compounds.
11
I. What is an Organic Compound?
- Contains carbon atoms
- Even though organic chemistry focuses on carbon,
many organic compounds also contain hydrogen (H),
nitrogen (N)-, oxygen (O), phosphorous (P) or
other elements. - Carbon molecules make up the bodies of all living
things and have many different functions - They are also commonly used in medicine, food,
paints, and gasoline.
12
Where is Carbon on the Periodic Table?
It forms 4 covalent bonds
13
(No Transcript)
14
Carbon has 4 electrons in its outmost electron
shell. (It has a valence of 4)
- it forms 4 covalent bonds
15
A. Carbon Bonding C atoms form many different
shaped molecules
- It can form straight chains, branched chains with
a single bond. - A carbon atom can also share two or even three
pairs of electrons with another atom.
16
Carbon bonds Chains, Branched, RingsEach line
represents a single covalent bond
17
Carbon also forms double triple bonds
A good site for more explanation
www.biologyjunction.com
18
Examples carbon bonding in molecules
19
B. Functional Groups - A specific group of atoms
that gives a known type of behavior to molecules
changes the characteristics of the compound-
(See page 52 in your textbook for more on
functional groups)
Hydrocarbons - C and H only Alcohols
- OH Acids - COOH Amines
- NH2
An excellent site to look more closely at
functional groups http//www.phschool.com/science
/biology_place/biocoach/biokit/function.html
20
(No Transcript)
21
More Functional groups
22
Example -adding a hydroxyl group makes ethane
into an alcohol-
- Example - adding an amino group
- - makes methane or ethane into- an amine
23
C. Naming Hydrocarbons
The simplest organic compounds are hydrocarbons.
Hydrocarbons are compounds that consist of
hydrogen and carbon atoms. When naming
hydrocarbons, the prefixes vary depending on the
number of carbons in a compound, the prefixes are
Number of Carbon(s) Prefix Number of Carbon(s) Prefix
1 Met- 6 Hex-
2 Eth- 7 Hept-
3 Prop- 8 Oct-
4 But- 9 Non-
5 Pent- 10 Dec-
http//chemwiki.ucdavis.edu/index.php?titleOrgani
c_Chemistry/Case_Studies/What_is_Organic3FCarbon
-containing_Compounds
24
Methane - the simplest carbon compound- 1
Carbon 4 hydrogen atoms
25
Other simple organic molecules
- Butane cyclohexane
26
D. Drawing Molecules ways that a molecule can be
drawn
- 1. Skeletal Structure (Kekulé Structure)
- In this form of representation, atoms are placed
on a plane and lines are drawn between atoms to
represent bonding electrons.
27
- 2. Condensed Structure
- A simplified version
- of the bond-line structure that omits the lines.
When there are 2 or more of the same kinds of
atoms attached to a central atom, a subscript is
used to indicate how many of these atoms are
attached. - 3. Lewis Structure
- valence electrons are represented
- as dots. This structure shows what
- atoms are bonded together, which electrons are
involved in bonding, lone pairs, any formal
charges.
28
E. Isomers- same chemical formula, different
structure!
- Organic molecules are three-dimensional
- the same set of atoms can be put together in
different ways, resulting in isomers - Example The atoms found in a simple sugar, with
the structural formula C6H12O6, can be arranged
in over a dozen different ways.
sonefe.org
29
F. Polarity Unequal electron sharing
- In covalent bonds e sharing is not always equal.
- Example Water - oxygen contains a higher
negative charge density than hydrogen. So
electron distribution is asymmetric, or polar,
and the oxygen atom is said to be
electronegative. - This asymmetry results in regions
- of slight negative positive
- charge in different regions of the
- molecule, denoted by the Greek
- symbol d (delta), for "partial" charge.
30
Electronegativity is a measure of the tendency of
an atom to attract a bonding pair of electrons
- Oxygen is a very electronegative atom pulls
electrons to itself. - That would leave the oxygen side of a molecule
fairly negative and the carbon fairly positive.
http//www.chemguide.co.uk/basicorg/bonding/eneg.h
tml
31
G. Sizes of Molecules
- 1. Monomers- small simple molecules
- 2. Polymers- big molecules formed when monomers
bonded to each other
32
3. Reactions to build or break down molecules
- Condensation Reaction
- 2 monomers join together- a water is released
- (an H from 1 end and an OH from the other
- end are cut loose when the monomers join.)
- Hydrolysis Reaction
- polymers are broken back down- they need a water
added.
33
Hydrolysis of sucrose
34
Condensation Reactions (also called dehydration
synthesis) -builds monomers into polymers
http//nhscience.lonestar.edu/biol/dehydrat/dehydr
at.html
35
Hydrolysis Reactions- break down polymers into
monomers by adding water
36
H. Energy from ATP
- Life needs a constant supply of energy
- Chemical bonds store energy.
- One molecule that living things use to store
energy is in the bonds of the ATP molecule - Adenosine Triphosphate
37
Adenosine Triphosphate
Blue ribose (a 5-carbon sugar)
Green adenine (a nitrogenous base)
Yellow phosphate groups Energy is stored in
bonds joining the phosphate groups
38
ATP-ADP Cycle.
The energy is released when the last P is taken
off
Energy is stored in ATP (ADP P) Energy is used
as needed ATP is converted back to ADP
phosphate.
39
ATP further explained
- A nitrogen-containing compound, adenine, is
represented by the two rings The three linked
phosphate groups, -PO4- are represented by the
small circles with a P. - Because the phosphate groups are close together
and have negative charges. When a bond between
the phosphate groups is broken, energy is
released. - This hydrolysis of ATP is used by the cell to
provide the energy needed to drive the chemical
reactions in an organism
40
II. Four Classes of Organic Molecules
- Much of biochemistry deals with the structures,
functions and interactions of cellular
components, basically - Carbohydrates
- Proteins,
- Lipids
- Nucleic Acids
41
Monomers Polymers
- Each group has small molecules (monomers)
- linked to form larger macromolecules
- (polymers) three to millions of subunits.
42
- Carbohydrates
- the most important energy source for cells
- short-term energy storage (sugar)
- intermediate-term energy storage
- starch for plants
- glycogen for animals
- as structural components in cells
- cellulose cell walls of plants
- chitin -exoskeleton of insects
43
Monomers Polymers
1. Monosaccharides - single sugar units
glucose 2. Disaccharides - two
monosaccharides. Lactose, maltose 3. Polysacc
harides - linking many sugar units
together Examples starch, glycogen,
cellulose
44
Carbohydrates
- General formula CH2On
- where n is a number between 3 and 6.
- Ex- glucose C6H12O6
45
Maltose Lactose are examples of disaccharides
What does it mean to be lactose intolerant?
46
Got Milk?- milks sugar is lactose
- Infant mammals are fed on milk from mom
- Enzyme lactase digests
- the molecule into its two
- subunits for absorption.
- in most species, including humans,
- the production of lactase gradually
- ceases with maturity, they are
- then unable to metabolize lactose
- becoming Lactose intolerant
47
A Polysaccharide
Lots of monomers linked together
48
B. Proteins
- Important as control and structural elements.
- Control enzymes, hormones.
- Structural -cell membrane, muscle tissue, etc.
- Amino acids are the
- building block of proteins
- All living things (and even viruses) use various
combinations of the same 20 amino acids.
49
1. An Amino Acid
an amino end (NH2) a carboxyl end (COOH).
R is the variable (R-group) of each amino acid.
50
(No Transcript)
51
Amino acids are linked together by joining the
amino end of one molecule to the carboxyl end of
another. Removal of water (condensation
reaction) links amino acids with a peptide bond.
52
2. PEPTIDE BONDS
53
Condensation reaction- (also called dehydration
synthesis) To build a peptide chain- add
monomers lose a water! Makes a peptide bond!
http//nhscience.lonestar.edu/biol/dehydrat/dehydr
at.html
54
- your amino acid pun for the day The cysteine
chapel.
http//popperfont.net/tag/biochemistry/
55
Some examples of proteins
- Antibodies they recognize molecules of invading
organisms. - Receptors part of the cell membrane, they
recognize other proteins, or chemicals, and
inform the cell... 'The Door Bell'. - Enzymes assemble or digest.
- Neurotransmitters and some hormones Trigger the
receptors... (the finger on the door bell...) - Channels, and pores holes in the cell membrane
(with or without a gate). Usually, filter the
flow...
56
3. Enzymes-
- Organic molecules that act as catalysts
- Enzymes substrates (the reactants) fit
together like a lock key - This fit weaken bonds so that less energy is
needed for reaction.
57
Enzymes- really important little guys!
- protein molecules, (or RNA molecules) can act as
biological catalysts -are essential for the
functioning of any cell. - Enzyme reactions depend on a physical fit between
the enzyme, at its active site on a specific
substrate. - the substrate and enzyme link together, it causes
a slight change in the enzymes shape, which
weakens some chemical bonds in the substrate,
that reduce the amount of activation energy
needed.
58
(No Transcript)
59
How Enzymes Work
- How Enzymes Work
- Most reactions in a cell require very high
temperatures to get going, which would destroy
the cell. Enzymes work by lowering the Activation
Energy of a reaction.
http//alevelnotes.com/Enzymes/144
- Most reactions in a cell require high
temperatures to start, which would destroy the
cell. Enzymes work by lowering the Activation
Energy of a reaction.
60
Examples
- Enzymes like DNA polymerase make important
molecules (like DNA) in cells. - Enzymes are used for a wide variety of purposes,
such as Pepsin Trypsin in digestion. - Enzymes are also used to destroy invading
Microorgansims. Phagocyte cells engulf pathogens
and the endocytosed - vesicle then fuses with Lysosomes
- which contain enzymes that destroy
- the pathogen's cell membrane.
61
tutorvista.com
62
Enzymes Need Optimal Temperatures -Unwind if too
hot, dont work if too cool.
- At high temperatures, enzymes denature (unwind)
and lose their catalytic properties. - at low temperatures, the reaction rate decreases.
63
Enzymes also need optimal pH!
- The pH at which enzymatic activity is maximal is
known as the optimum pH. - Within limits, enzymatic activity increases as
substrate concentration increases.
http//classes.midlandstech.com/carterp/Courses/bi
o225/chap05/ss2.htm
64
C. Lipids
- Functions
- Long-term energy storage.
- -Generally insoluble in polar substances (water)
- phospholipids are the major building block in
cell membranes - hormones ("messengers") play roles in
communications within and between cells.
65
Structure of Fatty Acids
- The carboxyl head is polar- therefore it is
HYDROPHILIC water loving - The hydrocarbon CH2 units are HYDROPHOBIC- water
fearing - (not water soluble).
66
Fatty acids
- Can be saturated (meaning they have as many
hydrogens bonded to their carbons as possible) - Unsaturated (with one or more double bonds
connecting their carbons, hence fewer hydrogens).
- A fat is solid at room temperature, while an oil
is a liquid under the same conditions. The fatty
acids in oils are mostly unsaturated, while those
in fats are mostly saturated.
67
2. Triglycerides
- Triglycerides are composed of three fatty acids
(usually) covalently bonded to a 3-carbon
glycerol.
68
(No Transcript)
69
(No Transcript)
70
Fats and oils function in energy storage.
- Animals convert excess sugars into fats.
- Most plants store excess sugars as starch,
although some seeds and fruits have energy stored
as oils (e.g. corn oil, peanut oil, palm oil,
canola oil, and sunflower oil). - Fats yield 9.3 Kcal/gm, while carbohydrates yield
3.79 Kcal/gm. Fats store six times as much energy
as glycogen.
71
Diets Fat Intake
- Attempts to reduce the amount of fats present in
specialized cells known as adipose cells that
accumulate in certain areas of the human body. - By restricting the intakes of carbohydrates and
fats, the body is forced to draw on its own
stores to makeup the energy debt. - The body responds to this by lowering its
metabolic rate, often resulting in a drop of
"energy level." - Successful diets usually involve three things
decreasing the amounts of carbohydrates and fats
exercise and behavior modification
72
3. Phospholipids
- One fatty acid is
- replaced with a
- phosphate.
- The negative charge(s) of the phosphate makes the
head of the phospholipid hydrophilic. The long,
hydrocarbon tail is non-polar and, therefore,
hydrophobic.
73
The water loving edge of the molecule orients
toward water- the inside and outside of the
cell. The water fearing edges of the molecule
orient toward each other to make a lipid
bilayer - the construction of the cell
membrane.
74
4. Cholesterol and steroids
- Structure is a lipid with 4 carbon rings with
various functional groups attached - Cholesterol has many biological uses, such as its
occurrence in the cell membranes, and its role in
forming the sheath of some neurons. Excess
cholesterol in the blood has been linked to
atherosclerosis, hardening of the arteries. - Steroids are mainly used as hormones in living
things
- Structure of four steroids. Image from Purves et
al., Life The Science of Biology, 4th Edition,
by Sinauer Associates (www.sinauer.com) and WH
Freeman (www.whfreeman.com), used with permission.
75
D. Nucleic Acids
- Function - informational molecules
heredity/genetic, protein synthesis, and energy - A nucleotide is formed from a 5 carbon sugar, a
phosphate and a nitrogen base. - Polymers formed by linking together long chains
of nucleotide monomers.
76
- 3 Nucleic Acids
- DNA-deoxyribonucleic acid
- Double strand of nucleotides
- Double Helix shape
- RNA-ribonucleic acid
- Single strand nucleotides
- ATP -Adenosine Triphosphate
77
Structure of DNA Structure of tRNA
-double strand of nucleotides -single
strand of nucleotides
78
(No Transcript)
79
RNA differs from DNA in the following ways
- RNA is single stranded while DNA is double
stranded. - RNA has a sugar called ribose while DNA has a
sugar called deoxyribose. - RNA has the base uracil while DNA has the base
thymine.
80
How DNA RNA work together
- DNA(deoxyribonucleic acid) is the genetic
material. - It functions by storing information regarding
the sequence of amino acids in each of the bodys
proteins. - This "list" of amino acid sequences is needed
when proteins are synthesized. - Before protein can be synthesized, the
instructions in DNA must first be copied to
another type of nucleic acid called messenger
RNA.
81
3 types RNA
- Messenger RNA, or mRNA.
- carries the code for building a protein from the
nucleus to the ribosomes in the cytoplasm. It
acts as a messenger. - Transfer RNA or tRNA.
- picks up specific amino acids in the cytoplasm
brings them into position on ribosome where they
are joined together in specific order to make a
specific protein. - Ribosomal RNA or rRNA place for protein synthesis
82
How a protein is built
83
(No Transcript)