Entropy in the Real World: Engines - PowerPoint PPT Presentation
Title:
Entropy in the Real World: Engines
Description:
Entropy in the Real World: Engines A heat engine, or more simply, an engine, is a device that extracts energy from its environment in the form of heat and does useful ... – PowerPoint PPT presentation
Number of Views:65
Avg rating:3.0/5.0
Title: Entropy in the Real World: Engines
1
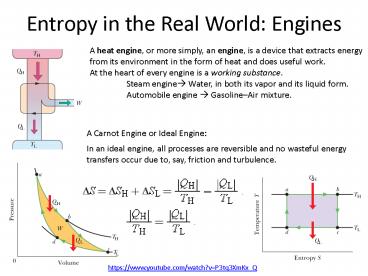
Entropy in the Real World Engines
A heat engine, or more simply, an engine, is a
device that extracts energy from its environment
in the form of heat and does useful work. At the
heart of every engine is a working substance.
Steam engine? Water, in both its vapor and its
liquid form. Automobile engine ? GasolineAir
mixture.
A Carnot Engine or Ideal Engine
In an ideal engine, all processes are reversible
and no wasteful energy transfers occur due to,
say, friction and turbulence.
https//www.youtube.com/watch?vP3tq3XmKx_Q
2
Efficiency of a Carnot Engine
3
Entropy in the Real World Refrigerators
A refrigerator is a device that uses work to
transfer energy from a low-temperature reservoir
to a high-temperature reservoir as the device
continuously repeats a set series of
thermodynamic processes. In a household
refrigerator, for example, work is done by an
electrical compressor to transfer energy from the
food storage compartment (a low-temperature
reservoir) to the room (a high-temperature
reservoir).
4
Problem 29, Page 557
Figure 20-27 shows a reversible cycle through
which 1.00 mol of a monatomic ideal gas is taken.
Assume that p 2p0, V 2V0, p0 1.01 105 Pa,
and V0 0.0225 m3. Calculate (a) the work done
during the cycle, (b) the energy added as heat
during stroke abc, and (c) the efficiency of the
cycle. (d) What is the efficiency of a Carnot
engine operating between the highest and lowest
temperatures that occur in the cycle? (e) Is this
greater than or less than the efficiency
calculated in (c)?