Two - Component Signal Transduction - PowerPoint PPT Presentation
Title:
Two - Component Signal Transduction
Description:
Two - Component Signal Transduction modular stimulas-response systems Response Regulator: conserved receiver domain + specific effecter domain Histidine Kinase Sensor ... – PowerPoint PPT presentation
Number of Views:230
Avg rating:3.0/5.0
Title: Two - Component Signal Transduction
1
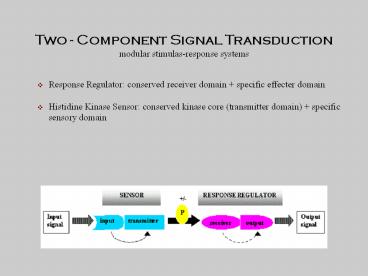
Two - Component Signal Transduction modular
stimulas-response systems
- Response Regulator conserved receiver domain
specific effecter domain - Histidine Kinase Sensor conserved kinase core
(transmitter domain) specific sensory domain
2
Phospo - transfer Reactions
The ?-phosphoryl group is transferred to the
conserved histidine side chain of the HK. The RR
catalyzes the transfer of the phosphoryl group
from the phospho-His residue to the conserved
aspartic acid side chain of the RR. Finally the
phosphoryl group is transferred from the
phospho-Asp residue to water in a hydrolysis
reaction
3
genomic distribution
- E. coli 30 HKs (5 hybrids) and 32 RRs
- Synechocystis sp 80
- Mycoplasma sp 0
- Bacillus subtilis 70
- Haemophilus influenza 9
- Helicobacter pylori 11
4
Histidine Kinase
- Most are periplasmic membrane receptors.
- Function as homodimers autophosphorylation is a
bimolecular event. - Periplasmic, N-terminal binding domain.
- Transmembrane domain.
- Linker domain.
- Histidine-containing phosphotransfer domain
- C-terminal kinase core.
- CheA NtrB are soluble, cytoplasmic HKs
5
Histidine Kinase
Kinase Catalytic Core 350 amino acids in
length dimerization domain ATP/ADP-binding and
phosphotransfer domain phosphatase activity found
in some
6
Histidine Kinase
Histidine-containing phosphotransfer domain 120
amino acids in length Histidine residue No kinase
or phosphatase activity
7
Histidine Kinase
- Sensing domain
- N-terminal domain that senses external stimuli
Usually periplasmic receptor - not always - In many cases the ligand or stimulas is unknown
- Little or no sequence similarity.
- Transmembrane and Linker Domains
- Poorly understood
- Critical for propagation of signal from
periplasmic binding domain to kinase core
8
Response Regulator
- N-terminal Receiver or Regulatory domain
- C-terminal Effector domain DNA-binding
transcriptional regulator - enzymatic activity (CheB or RegA)
- protein-protein interactions
- Catalyze the transfer of phosphryl group from
phospho-HK to conserved aspartic acid
phosphorylation results in conformational change
of response regulator. - Many also catalyze auto-dephosphorylation.
9
Modular Organization of tcs
E. coli osmoregulation
E. coli Anoxic Redox Regulation
E. coli chemotaxis
B. subtilis sporulation
10
Modular Organization of tcs
- Phosphotransfer Systems His --gt Asp
- Phosphorelay Systems His --gt Asp --gt His --gt Asp
Added complexity provides for multiple regulatory
checkpoints and points of integration between
signaling pathways
11
Regulatory Mechanisms The whole point of signal
transduction is regulation. The signaling
pathway provides steps at which the flow of
information can be modulated.
- Regulation of the Histidine Kinase
- Autokinase activity either stimulated or
repressed by specific stimulas. - RR phosphatase activity of the histidine kinase
can be modulated. - Regulation of the Response Regulator
- Phosphorylation by cognate HK
- Dephosphorylation by specific phosphatases
- Stimulation of intrinsic autophosphatase
activity. - Inhibition of phosphotransfer
- Regulation of the expression of the two-component
proteins.
12
Integration of Signals i
- Five related HKs are capable of phosphorylating
the RR SpoOF (KinA, KinB, KinC, KinD and KinE). - KinA, KinB, KinC, KinD and KinE share sequence
similarities surrounding the phosphorylatable
histidine residue but differ in their sensing
domains. - RapE is expressed during vegetative growth.
- RapA and RapB are induced by the ComA/ComP TCS
- Therefore sporulation is prevented during
vegetative growth and competence development
13
Integration of Signals II
- ResD/ResE regulates expression of genes required
for anaerobic respiration. - PhoP/PhoR regulates expression of genes required
for phosphate uptake. - When phosphate is low, phosphorylated PhoP
induces expression of res operon while repressing
the PhoP-independent promoter. - Phosphorylated ResD activates phoP-phoR
expression (positive feedback loop)
14
Integration of Signals III
- The product of the udg gene is required for both
the Pmr-regulated modification of LPS and the
Rcs-dependent production of capsule. - Both PmrA and RcsB can bind and activate
transcription from the ugd promoter. - PmrD activates PmrA post-transcriptionally
independently of PmrB in response to Mg. - The ugd gene is expressed in response to Mg,
Fe OR cell envelope stress.
15
- High osmotic pressure changes the conformation of
the outer segment of EnvZ sensor protein. - The change is transmitted inwards and EnvZ
phosphorylates itself using ATP. It then
transfers the phosphate group to OmpR. The OmpR-P
form binds DNA.
16
- When OP is low, there is only a trace of OmpR-P,
but this is sufficient to bind to the high
affinity site in front of the ompF gene and
activate transcription. - At high OP, the concentration of OmpR-P rises and
it can now occupy the low affinity sites. This
stops transcription of the ompF gene and
activates transcription of the ompC gene. - In addition the micF gene is transcribed to give
MicF RNA. This binds to the front of the ompF
message and prevents translation. Thus whenever
expression of ompC is increased, expression of
ompF is decreased. (Actually micF is more
probably important for temperature control than
for osmoregulation.)
17
- CheA is HK that phosphorylates RRs CheY and CheB.
- Phosphorylation of CheA stimulated by unoccupied
receptors (requires CheW). - Phosphorylated CheY binds the flagellar motor and
stimulates CW rotation of the motor which results
in enhanced tumbling. - CheZ is a phosphatase that dephosphorylates CheY
- Upon phosphorylation by CheA, CheB removes methyl
groups from MCP resulting sensory adaptation. - Ligand bound MCP undergoes conformational change
that inhibits autophosphorylation of CheA.
18
Cyclic-di-gmp-mediated regulation in bacteria
The discovery of c-diGMP dates back to work
published by Moshe Benziman on the regulation of
cellulose biosynthesis in Gluconacetobacter
xylinum (formerly called Acetobacter xylinum) and
Agrobacterium tumefaciens. In two landmark
papers, published in 1987 and 1998, Benziman and
colleagues first described the identification of
c-diGMP as an allosteric regulator of cellulose
synthase (CS) CS activity is almost completely
dependent on the presence of c-diGMP
19
(No Transcript)
20
(No Transcript)
21
diguanylate cyclase
diguanylate phosphodiesterase
2 GTP
c-di-GMP
2 GMP
2 PPi
EAL
GGDEF
22
Cyclic di-GMP as a Bacterial 2nd Messenger
diguanylate cyclase
diguanylate phosphodiesterase
c-di-GMP
2 GMP
2 GTP
2 PPi
EAL
GGDEF
EAL
Activity of Effector Protein
23
(No Transcript)
24
(No Transcript)
25
BrkA
Fimbrea
FHA
Tracheal Colonization Factor
Pertactin
Ptl
Adenylate Cyclase Toxin
Pertussis Toxin
Tracheal Cytotoxic Toxin
26
Vrg18
Vrg73
Vrg6
BvgAS
Bvg
Bvg-
25oC Nicotinic Acid MgSO4 DbvgAS
27
H
BvgS
D
H
D
BvgA
HTH
28
37oC
ATP
ATP
H
H
ADP
ADP
BvgS
D
D
D
H
H
D
BvgA
HTH
29
37oC
ATP
ATP
H
H
ADP
ADP
BvgS
D
D
P
D
H
H
D
BvgA
HTH
30
37oC
ATP
ATP
H
H
ADP
ADP
BvgS
D
D
D
H
P
H
D
BvgA
HTH
31
37oC
ATP
ATP
H
H
ADP
ADP
BvgS
D
D
D
H
H
Virulence genes
D
BvgA
HTH
32
25C or 37C SO or Niacin
H
H
BvgS
D
D
D
H
H
D
HTH
Virulence genes
BvgA
33
bvgR
bvgA
bvgS
AUG
AUG
GUA
P
P
BvgA
BvgS
P
BvgR
vrg6
P
vrg18
P
vrg24
P
vrg53
P
vrg73
34
MgSO
4
?
-
Nicotinic Acid
Temperature
BvgS
RisS
BvgA
RisA
BvgR
35
Cyclic di-GMP as a Bacterial 2nd Messenger
diguanylate cyclase
diguanylate phosphodiesterase
c-di-GMP
2 GMP
2 GTP
2 PPi
EAL
GGDEF
EAL
Activity of Effector Protein
BvgR
EAL
36
(No Transcript)
37
GGDEF/EAL proteins in Bacillus anthracis
BA 0548
EAL
BA 0628
EAL
BA 2533
EAL
BA 3879
EAL
BA 4203
EAL
BA 4263
BA 5543
EAL
BA 5593
EAL
BA 5664
38
Virulence of GGDEF/EAL Mutants
gevA GGDEF/EAL virulence regulator A
39
Growth of gevA Mutant
40
GevA
PAS domains act as sensory modules for oxygen
tension, redox potential or light intensities.
The domain functions through protein-protein
interactions or through binding cofactors within
their hydrophobic cores to regulate
protein-protein interactions in response to
stimuli.
41
GevA
AAAAA
AAL























![[VII]. Regulation of Gene Expression Via Signal Transduction PowerPoint PPT Presentation](https://s3.amazonaws.com/images.powershow.com/4917995.th0.jpg?_=20131220041)






