THERMAL OXIDATION - Chapter 6 - PowerPoint PPT Presentation
1 / 45
Title:
THERMAL OXIDATION - Chapter 6
Description:
THERMAL OXIDATION - Chapter 6 Basic Concepts SiO2 and the Si/SiO2 interface are the principal reasons for silicon s dominance in the IC industry. – PowerPoint PPT presentation
Number of Views:375
Avg rating:3.0/5.0
Title: THERMAL OXIDATION - Chapter 6
1
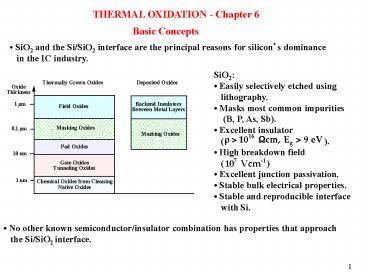
THERMAL OXIDATION - Chapter 6
Basic Concepts
SiO2 and the Si/SiO2 interface are the
principal reasons for silicons dominance in
the IC industry.
SiO2 Easily selectively etched using
lithography. Masks most common impurities
(B, P, As, Sb). Excellent insulator
( ). High breakdown field ( )
Excellent junction passivation. Stable bulk
electrical properties. Stable and reproducible
interface with Si.
No other known semiconductor/insulator
combination has properties that approach the
Si/SiO2 interface.
2
Thermal Oxidation and SiO2 Interface
Applications
SiO2 grows on Si (also _at_ RT) enables very easy
IC formation ensures stability and reliability.
Lower thermal budget 1 2 nm
New dielectrics ? ? to avoid tunneling. (high
K)
Low K dielectrics
3
Historical Development and Basic Concepts
Oxide growth using O16 and O18 isotopes
identifies the mechanism.
Neutral O2 and H2O and/or OH are dominant species
in oxidation, not atoms or ions O, O- , O2-,
Volume of SiO2 is 30 larger than Si.
(1.3)3 2.2 volume of the oxide cannot be
accommodated in Silicon
Oxidation involves a volume expansion (
2.2X). Especially in 2D and 3D structures,
stress effects play a dominant role.
4
Silicon Consumption During Oxidation (LOCOS)
Birds Beak formation Stress at the Si/Si3N4
interface
5
Structure of Silica Glass
Short range order maintained
Amorphous material
hydrogen
Non-bridging oxygen in fused Silica (not present
in crystalline SiO2)
Network modifier ----gt Qm
Si can be replaced by deposits. B,P,As or Sb
network formers.
- large compressive stress (5109 dynes/cm2)
exists in SiO2. High temperature can relief
stress by viscous flow. - Large difference in the thermal expansion
coefficients of Si and SiO2.
Silicon in tension ? refer to curvature
6
SiO2 is amorphous even though it grows on a
crystalline substrate.
(Intel Web site)
Four charges are associated with insulators
and insulator/semiconductor interfaces.
Qf - fixed oxide charge Qit -
interface trapped charge Qm - mobile
oxide charge Qot - oxide trapped charge
7
SiO2/Si System Structure and Charges
(Fig 3.15) amorphous/crystalline interface
flat. (TEM) Roughness ? with ? growth rate and ?
T.
Detect density at the interfaces is 109 1011
cm-2.
Trapped charges Qot important when isolation
radiation is present (space applications, plasma
processing) EPROM
Fixed charge 109 1011 cm-2 is and does
not change in device operation.
Interface charge traps due to dangling Si bonds
? change in operation Qp?Qit both related to
unoxidized Si atoms.
Deal triangle
Reduce charges since they degrade device
operation ? ? T , H2 anneal..
8
Oxide Charges and Their Annealing
H fast through SiO2
Al annealing AlOH ? ALOH
t to reach equilibrium ?with T
Qit? H2 ? 2H ?SiH??SiH
Increasing surface roughness increases charges
(100) Si used in ICs
Ar better - does not react with Si.
9
(No Transcript)
10
Oxidation systems are conceptually very
simple. In practice today, vertical furnaces,
RTO systems and fast ramp furnaces all find
use.
LOCOS or STI
Gate Oxides
DRAM Dielectrics
Thermal oxidation can potentially be used in
many places in chip fabrication. In
practice, deposited SiO2 layers are
increasingly being used (lower Dt).
11
Manufacturing Methods and Equipment
Vertical furnaces are also used. Better
uniformity, easier automation, cleaner - no
contact with the tube
Wafer loading should use cantilever or elavators
(perpendicular) to avoid touching the walls.
3 zones
0.5 C
Dry or wet oxidation
Ramping of T from/to 800 C ( 10 C/sec) Add HCl
or TCA for gettering purpose (metals, Na )
_at_ 1000 C, in water,
? 2X for each 100 C
Temperature control 0.5 C
12
(No Transcript)
13
Measurement Methods
- Physical (Destructive) Etch a step and measure
(stylus, microscope with monochromatic light, - AFM, SEM, TEM)
- Optical (usually non destructive) thick oxides
(color chart, ellipsometry, reflectance) but for
thin oxides ellipsometry
REFLECTANCE
white or monochromatic light
Refraction indexes
Color chart (xox gt 50nm) gt not destructive
interference will affect the reflected light gt
color correlated with thickness of a dielectric
layer (10-20 nm accuracy) - use standards
For monochromatic light minima and maxima in the
reflected beam allow to determine xox (fringes,
spectrometers with sweeping wavelength l for
fixed ? we can find extrema)
Good for a few tens of nm
Ellipsometry uses polarized light and detect the
change in polarization of the reflected light due
to a film (thickness, index of refraction)
1,2,3 max 1/2 min
14
Powerful technique for characterizing
semiconductor/ insulator structures.
Electrical Measurements
C-V Measurements
MOS Capacitor
accumulation
Capacitance-voltage method
ac signal
xox obtained
Charge Density
depletion
Inversion Equilibrium conditions.
Nd obtained
15
CV Measurements
Low frequency (1Hz), high frequency (100Khz
11) AC signals used for C-V Measurements.)
QI follows QG ? C COX
XD XDmax?QD fixed
XDgt XDmax
Holes generated in the D.L and attracted by the
gate source the DL when VG increases
High frequency AC signal changes faster than QI
can respond (generation is slow)
To avoid deep depletion
16
Bias-Temperature (BT) Test
17
Charges Derived
Qm, Qot have similar effect as Qf (shift
characteristics)
P-type
Due to traps
Eit?Ec
States near Ec
Qi present Eit ? Ev
Midgap states
Traps cannot charge or discharge - do not respond
to HF signal
States near Ev
Always present
Qi respond to DC voltage stretch out ? change
in EF (VG), charges at Eit.
Stress of the oxide (ex. charge injection,
radiation) ? C-V degradation (? time to
breakdown, charge to breakdown)
18
Experiment Why no C-V change is observed?
- Assume good oxide/Si interface.
- Masking oxide was too thin ? high doping under
the gate (CD ??) - Supply of carriers would give, C-VHF C- VLF
19
Models and Simulation First -Order Planar Growth
Kinetics - Linear Parabolic Model
Boundary layer
In steady state F1F2F3
Deal and Groove Model
Reaction at Si surface
Henry law
CCO (PGPS)
Oxidant solubility in SiO2
h ??
hG- mass transfer coefficient
Gradient Co?Ci
hhG/HkT
Transport of the oxidant to the oxide surface.
Transport to Si Diffusion of O2 (H2O) through
the oxide
Sol.sol. in SiO2
ksx0/D ltlt1 or ksx0/D gtgt1
20
Models and Simulation First Order Planar Growth
Kinetics Linear Parabolic Model
transport
reaction
ksx0/D ltlt1 CI ? C Diffusion fast compared to
chemical reaction for thin oxides.
ksx0/D gtgt1
Fast reaction - diffusion limits oxidation (thick
oxides)
Fitting parameters
CI ?0
xo(t)
General dependence of oxide thickness on time.
ksDf(t)
Transition region _at_about 50-200 nm
Linear rate constant
parabolic Rate constant
Thin oxides Thick oxides
21
Linear and Parabolic Rate Constants
Experimental results
Derivation of A B
Arrhenius dependence
Parabolic
Linear
Represent Si-Si bond breaking
activation energies
E2
Linear
Parabolic
Breaking Si bonds
diffusion
parabolic
E1
Represent diffusion of O2 or steam
BwetgtBdry because Cwet gtgt Cdry _at_1100 C,
31019 cm-3 gtgt 51016 cm-3 !!
22
Wet oxidation - used for thicker oxides
Dry oxidation - used up to 100-200 nm
CwetgtgtCdry
c)
a)
b)
Calculated (100) silicon dry O2 oxidation rates
using Deal Grove.
Calculated (100) silicon H2O oxidation rates
using Deal Grove.
Example Problem 6.13 in the text a) 3 hrs in
O2 _at_ 1100 C 0.21 µm b) 2 hrs in H2O _at_
900 C 0.4 µm c) 2 hrs in O2 _at_ 1200 C 0.5
µm total oxide thickness.
23
Thin Oxide Growth Kinetics
A major problem with the Deal Grove model was
recognized when it was first proposed - it
does not correctly model thin O2 growth
kinetics. Experimentally O2 oxides grow much
faster for 20 nm than Deal Grove predicts.
MANY models have been suggested in the
literature.
1. Reisman et. al. Model
(17)
Power law fits the data for all oxide
thicknesses. a and b are experimentally
extracted parameters. Physically - interface
reaction controlled, volume expansion and viscous
flow of SiO2 control growth.
2. Han and Helms Model
(18)
Second parallel reaction added - fits the
data for all oxide thicknesses. Three
parameters (one of the A values is 0). Second
process may be outdiffusion of OV and reaction at
the gas/SiO2 interface.
24
3. Massoud et. al. Model
(19)
Second term added to Deal Grove model - higher
dx/dt during initial growth. L 7 nm, second
term disappears for thicker oxides. Easy to
implement along with the DG model, used in
process simulators. Data agrees with the
Reisman, Han and Massoud models. (800C dry O2
model comparison below.)
25
Other Models for Planar Oxidation Kinetics -gt
Incorporate Thin Oxides
linear
D-G model fails For thin oxideslt 20nm
- Models
- O2- coupled diffusion with holes
- thermionic emission of e- from Si
- micropores, channels - parallel transport
- parallel oxidation with O2, O (diffusion and
reaction) - silicon surface with additional sites
800 C
Volume expansion at the interface provided by
viscous flow - model applicable to 2D oxidation
in nonplanar structures
a,bf(T, p.press.)
1)
2)
O2 through SiO2, O2 O _at_Si parallel reactions O
dominates _at_low T O2 dominates _at_ high T
1000C
B/A a Pn
3)
n1 H2O, n.5 Dry O2
4)
Massoud et al. L7nm Good for thin oxides
Added for thin oxides
26
Effect of Volume Mismatch in Si/SiO2 System
Recessed LOCOS
H2O_at_1000C Find time to get planar surface?
Example
2.2X volume expansion -gt 45yoxySi so
yoxySi/.45
ySi
Total oxide thickness to be grown
yoxySi/0.45ySi0.5µm
ySi0.41µm yox0.91µm
For H2O
Time for dry oxidation would be unrealistically
long
27
Dependence of Growth Kinetics on Pressure
Dry O2
CaPG
if
B, B/A ?PG ? dxo/dt ?PG
0.1 atm
D-G model gives good match for wet oxidation not
for dry - linear-parabolic model is incomplete
B?P B/A?Pn 0.5ltnlt1.0
Many models use modified Si/SiO2 interface
reaction - none is widely accepted
10 atm
D-G model with correction B/A(B/A)iP
B(B)iP for H2O) B/A(B/A)iPn B(B)iP for O2
n0.7-0.8
28
Mixed Ambient Growth Kinetics
Role of Cl in Oxidation Processes
Mixed ambient
B/A
B
f(HCl)
Two terms include independent reactions for H2O
and O2 (not a good model) H2O/HCl (first term
only) and O2/HCl interaction between oxidants -
dependence -gt pressure role
29
Orientation Effects in Oxidation
wet
dry
(100), (111), and Polysilicon
Density of atoms (bonds) in (111)gt(100)
No effect of orientation in the parabolic
regime Very thin oxide growth at high pressure
and low T (ex. 800C) (100) gt(111)
In the linear regime
Related to stress
Simulated oxide growth
30
C. 2D SiO2 Growth Kinetics
These effects were investigated in detail
experimentally by Kao et. al. about 15 years
ago. Typical experimental results below.
(Kao et.al)
31
2D SiO2 Growth Kinetics
Difference in volume -gt problems when expansion
is restricted (SiO2 confined)
- Experiments by Kao et al.
- Retardation at sharp corners (2X for 500 nm SiO2)
- Retardation larger _at_ low T (no effect _at_ 1200 C)
- Interior (concave) corners oxidize slower than
exterior (convex) but both slower than flat Si - Reasons
- Crystal orientation
- Diffusion of oxidant through amorphous SiO2 is
the same -gt no dependence on direction - Stress (volume difference) SiO2 under large
compressive stress -gt affect both oxidant
transport and reaction at the Si surface
Poly-Si for contrast
32
In addition, the flow properties of the SiO2
need to be described by a stress dependent
viscosity
(23)
Where is the shear stress in the oxide and
VC is a fitting parameter. SUPREME uses many
fitting parameters.
These models have been implemented in modern
process simulators and allow them to predict
shapes and stress levels for VLSI structures
(above right). ATHENA simulation Left - no
stress dependent parameters, Right - including
stress dependence.
33
Oxidation of Non-Planar Structures
Example
Stress retards oxidation _at_high T viscoelastic
flow relaxes stress Oxide viscosityf(stress, T)
Stress _at_?Tgt Stress _at_?T
no stress
Stress included
(outer)
Reaction diffusion solubility
(inner)
Viscoelastic properties (nonlinear in T) of
poly-Si, Si3N4 also have to be included in
simulations of oxidation History of Stress VERY
IMPORTANT Stress in an oxide depends on growth T.
In sequential processing, transient will appear
in the next step _at_ higher T from the original
stress (higher at lower T) which sets the oxide
growth rate below that at high T (lower stress).
stress dependent activation values
Also TF(irst)gtTS(econd) StressFltStressS GrowthFgtGr
owthEquilibrium
- Relieve stress
- High T - not good for ICs
- O2/NF3 ?
- Corona discharge ?
34
Segregation of Dopants at the Si/SiO2 Interface
B
B in H2
Dry O2
Ga - not useful as a dopant
P, As
35
Point Defect Based Models
The oxidation models we have considered to this
point are macroscopic models (diffusion
coefficients, chemical reactions etc.).
There is also an atomistic picture of
oxidation that has emerged in recent years.
Most of these ideas are driven by the volume
expansion occurring during oxidation and the
need for free volume.
In Chapter 3 we described internal oxidation in
the following way
Oxidation Enhanced Diffusion (OED) Oxidation
Retarded Diffusion (ORD) Both are related to the
interface reaction (B/A or ks) Si/SiO2 -
generation of I 1/103 Si atoms diffuse to Si,
others oxidized
Surface oxidation can be thought of in the same
way.
36
The connection between oxidation and other
processes can then be modeled as shown below.
Increase in concentration of Si-I -gt OED also
laterally
ATHENA simulation of OED.
Original B layer was uniform
Oxidation injects interstitials to create free
volume for the oxidation process. Oxidation
can also consume vacancies for the same reason.
These processes increase I concentrations and
decrease V concentrations in nearby silicon
regions. Any process (diffusion etc) which
occurs via I and V will be affected.
37
Advanced Point Defects Based Model for Oxidation
Relation to dopant diffusion
Interstitials (I) diffuse far
G-R determine a net flux of I -gt effect on
diffusion even in a buried layer.
- Experimental G-R for various layers (both depend
on surface layer) - Reasons
- kinks on the surface
- generation/surface
- regrowth/segregation/Si/SiO2
38
Substrate Doping Effects Concentration Enhanced
Oxidation (CEO)
5x faster due to dopant
2x faster
NDopant P -gt oxide growth by ? B/A not by B
especially _at_ low T about 3-4X ? due to ?
concentrations Properties of oxide do not change
for P but change for B
Low T
High T
- Oxidation needs V for volume expansion so for ?
dopant concentrations, charged V ?(V-, V -
N-type V - P -type) -gt ? B/A - Dopant segregation N -gt to Si
- P -gt SiO2
- Interface changes during oxidation
- -gt growth rate changes
CEO stronger for N than P (B/A grows, B does
not) CEO for Boron changes B but not B/A due to
incorporation in the oxide
39
Recessed LOCOS - Simulation
40
Complete Process Simulation of Oxidation
Many of these models (and others in Chapter 6),
have been implemented in programs like
SUPREM.
Simulation of an advanced isolation
structure (the SWAMI process originally
developed by Hewlett-Packard), using
SSUPREM IV. The structure prior to oxidation
is on the top left. A 450 min H2O oxidation
at 1000 C is then performed which
results in the structure on the top right. An
experimental structure fabricated with a
similar process flow is shown on the bottom
right. The stress levels in the growing SiO2
are shown at the end of the oxidation on
the bottom left.
41
Silicide Oxidation - Similar to Silicon
Silicides (TiSi2, CoSi2) used in ICs instead of
polysilicon (400 µ?cm) local interconnects
Oxidation results in formation of SiO2 (for most
metals) or mostly MOx for HfSi2, ZrSi2, TiSi2
Metal bonds break -gt M diffuses to the Si
(polysilicon) substrate, released Si form SiO2
Bsilicide Bsilicon B/AsilicidegtgtB/Asilicon
For TiSi2 Si atoms diffuse from the poly_Si For
CoSi2, NiSi2, PtSi - metals diffuse to poly_Si
Parabolic growth
42
Polysilicon Oxidation
Rate larger than (100) because of various
orientations and sizes of grains -gt B/Af(grain
size) not B
Grain boundary important in doping, regrowth at
high T
Si3N4 Growth and Oxidation Kinetics
Niride is a mask against in LOCOS
Si3N4 growth slow in NH3 2.5-7.5 nm _at_900C
or Si2N2O
Kooi effect
Small diffusion of oxidants through Si3N4
43
Oxidation Induced Stacking Faults (Preferential
Etching)
OISF ? with time and temperature of the oxidation
process
Preferential etching reveals S-pits and OISF
Surface and bulk OISF
Increasing etching time
44
Formation and Annihilation of OISF in Wet and Dry
Oxidation Processes
OISF depend on available I at the Si surface
Wet
Dry Oxidation
45
Summary of Key Ideas
Thermal oxidation has been a key element of
silicon technology since its inception.
Thermally, chemically, mechanically and
electrically stable SiO2 layers on silicon
distinguish silicon from other possible
semiconductors. The basic growth kinetics of
SiO2 on silicon are controlled by oxidant
diffusion and Si/SiO2 interface chemical
reaction. This simple Deal-Grove model has
been extended to include 2D effects, high
dopant concentrations, mixed ambients and thin
oxides. Oxidation can also have long range
effects on dopant diffusion (OED or ORD)
which are modeled through point defect
interactions. Process simulators today
include all these physical effects (and more) and
are quite powerful in predicting oxidation
geometry and properties.