Enzyme Stereospecificity - PowerPoint PPT Presentation
1 / 11
Title:
Enzyme Stereospecificity
Description:
An enzyme oxidizing a-hydroxy acids to a-keto acids may bind only one enantiomer (binding specificity) or it may bind both but oxidize only one isomer. ... – PowerPoint PPT presentation
Number of Views:1047
Avg rating:3.0/5.0
Title: Enzyme Stereospecificity
1
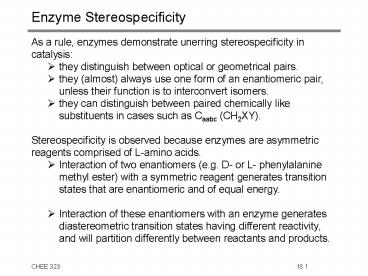
Enzyme Stereospecificity
- As a rule, enzymes demonstrate unerring
stereospecificity in catalysis - they distinguish between optical or geometrical
pairs. - they (almost) always use one form of an
enantiomeric pair, unless their function is to
interconvert isomers. - they can distinguish between paired chemically
like substituents in cases such as Caabc (CH2XY). - Stereospecificity is observed because enzymes are
asymmetric reagents comprised of L-amino acids. - Interaction of two enantiomers (e.g. D- or L-
phenylalanine methyl ester) with a symmetric
reagent generates transition states that are
enantiomeric and of equal energy. - Interaction of these enantiomers with an enzyme
generates diastereometric transition states
having different reactivity, and will partition
differently between reactants and products.
2
Enzyme Specificity
- Enzymatic catalysis always involves prior complex
formation between the reactant and the enzyme - The substrate binds to a specific region on the
enzyme in every catalytic cycle, and catalysis
only occurs at the active site. - Specificity can be imposed on the binding step,
on any subsequent catalytic step, or both. - An enzyme oxidizing a-hydroxy acids to a-keto
acids may bind only one enantiomer (binding
specificity) or it may bind both but oxidize only
one isomer. - The active site of an enzyme consists of an array
of amino acid functional groups (and in some
cases coordinated metals) positioned in a
specific three dimensional orientation.
3
Enzyme-Substrate Interactions
- The enzyme hexokinase catalyzes a phosphoryl
transfer from ATP to C-6 of glucose - Conformational changes in the tertiary
- structure of hexokinose resulting
- from its binding of glucose have
- been observed. This is called
- induced fit, where conformational
- changes in the flexible enzyme
- when bound to a substrate
- generates a transition state
- structure (enzyme-substrate)
- of relatively low energy.
4
Conceptual Models of Enzyme-Substrate Binding
- Fischer lock and key hypothesis
- Koshland induced-fit hypothesis
- Hypothesis involving strain or transition state
stabilization
5
Model Reactions Strain and Distortion
- Inducing strain in the substrate and release of
that strain in the transition state to products
is known to increase the rate of many classes of
reactions. - A model reaction that illustrates this effect is
the phosphate-ester hydrolysis of cyclic and
acyclic analogues - Under similar conditions the cyclic ester (I)
hydrolyzes at a relative rate that is 108 times
faster than the acyclic ester (II). - Distortion and strain imposed by an enzyme on a
bound substrate is expected to destabilize it
relative to the transition state, thereby
enhancing the rate of reaction.
6
Model Reactions Proximity Effects
- Enzyme literature makes reference to catalysis by
approximation, referring to the capacity of
enzymes to make reactants proximal - adjacent to
each other. - An increase in the effective concentration of
reactants over that found in the bulk of the
solution is expected to increase the rate of
reaction - Model compound studies demonstrate this effect by
comparing rates for similar intra- versus
intermolecular transformations. - Imidazole-catalyzed hydrolysis of p-nitrophenyl
acetate relates to an enzyme active site
involving a histidine residue - The bimolecular rate constant under a given set
- of conditions is kobs,1 35 min-1M-1 for this
reaction.
7
Model Reactions Proximity Effects
- To assess the acceleration derived from
covalently imposed proximity, the intramolecular
analogues of this reaction were studied. - The first-order rate
- constant for this
- unimolecular case
- under the same
- conditions was
- kobs,2 200 min-1.
- An effective molarity of imidazole in this
intramolecular reaction can be calculated through
a comparison of the rate constants - kobs,2 kobs,1 Imidazoleeff
or Imidazoleeff 5.7 M - Inclusion of a third methylene group
- to permit a 6-member transition
- state enhances the rate further,
- increasing Imidazoleeff to 23.9 M.
8
Enzymes Transition State Theory
- Our treatment of closed catalytic reaction cycles
usually includes the assignment of a rate
determining step. Assuming this step is
elementary, we can discuss its kinetics in terms
of transition state theory. - Depending on the reaction mechanism, catalysis
influences reaction rates by. - 1. increasing the concentration of activated
complex - Acid/base catalyzed organic transformations
- 2. providing an alternate reaction pathway
requiring a lower energy transition state - Organotransition metal catalyzed isomerization of
olefins - 3. Destabilizing the substrate through
complexation - Enzyme catalyzed organic transformations
9
Kinetics of Enzyme Catalyzed Reactions
- In spite of the apparent complexity of enzyme
catalyzed processes, their reaction kinetics can
be described by a relatively simple sequence of
reactions which, while not elementary, are
sufficient to account for the observed behaviour. - Michaelis and Menten (1913)
- If we assign the second reaction as irreversible
and rate determining, - where,
- r reaction rate (mole l-1 s-1)
- S concentration of substrate (mole l-1)
- ET total enzyme concentration (mole l-1)
- Ke Equilibrium constant (l mole-1)
10
Kinetics of Enzyme Catalyzed Reactions
- Michaelis-Menten rate expressions are usually
expressed in an alternate form - where,
- Vmax k2ET (s-1)
- S concentration of substrate (mole l-1)
- Km Michaelis constant (mole l-1) 1/Ke
- Suppose an enzyme concentration of 510-7 mole
/litre can support a reaction rate of, - As we vary the concentration of substrate from
0.001 M to 1.0 M, how does the ratio of ES /
E respond?
11
Kinetics of Enzyme Catalyzed Reactions
- It is clear that a relatively small substrate
concentration will bind a very high fraction of
enzyme. - Even more impressive is the activity of this
enzyme-substrate - complex, which allows just 0.55 mmoles/litre to
support a reaction velocity of 310-5 s-1.