Binding - PowerPoint PPT Presentation
1 / 15
Title:
Binding
Description:
1. Binding. Old: based on mass action. various definitions of equilibrium constants ... Titration curve for ribonuclease at 25 C and three ionic strengths. ... – PowerPoint PPT presentation
Number of Views:25
Avg rating:3.0/5.0
Title: Binding
1
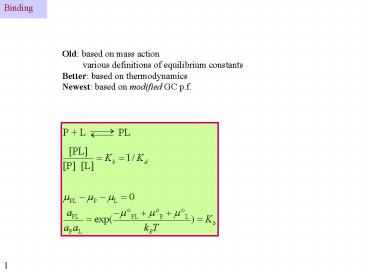
Binding
Old based on mass action various definitions of
equilibrium constants Better based on
thermodynamics Newest based on modified GC p.f.
1
2
Binding
Need Ligand activity (M is dilute and treated
as ideal solution) saturation ML/(MML)
2
3
Binding
Need Ligand activity saturation
ML/(MML)
- Spectra of M and ML differ
- Excess L is present as ML
3
4
Binding
Single-site binding curves
4
5
Binding to macromolecules
Multiple-site binding Independent and not
equivalent Independent and equivalent DEPENDENT
Second site with lower affinity
5
6
Net charge 18
COOH ? COO H HISH ? HIS H Phe-OH
? Phe-O H NH4 ? NH3 H
High ionic strength
low
Number of H ions titrated per molecule
Net charge 11
pH
Titration curve for ribonuclease at 25C and
three ionic strengths. (from Tanford and
Hauenstein, JACS, 1956)
6
7
Binding to macromolecules
Multiple-site binding n independent and
equivalent sites
(unfortunate distinction) 1. Site binding
constant sites are distinct Stoichiometric
binding constants 2. for one site 3. for i
sites
7
8
Binding to macromolecules
Fit model to data Experiment ltiLgtexpt , ln
aL,expt Model binding constants,
dependencies Compute ltiLgtmodel for each ln
aL,expt Least squares fit minimize S(ltiLgtmodel
ltiLgtexpt)2 May weight points according to their
reliability
8
9
Binding to macromolecules
Multiple-site binding dependent sites
1
2
Simple case
1
2
1
2
1
2
Often linked to a conformation change (o to o)
9
10
Binding linked to conformation change
Symmetrical model has species A, AL, AL2, B, BL,
BL2
10
11
Thermodynamic approach to interactions of small
molecules with macromolecules
e.g., Large M and L cannot occupy same volume (L
can be a coiled H2O soluble polymer like PEG)
reference
exclusion
binding
L
L
L
L
Also different L in these two situations!
constant mL
observation is n dNL/dNM ? 0 to find a general
formulation in terms ofthermodynamics or
statistical mechanics, i.e., model-free.
11
12
Thermodynamic approach to interactions of small
molecules with macromolecules
And the other way around
12
13
Thermodynamic approach to interactions of small
molecules with macromolecules
I wish to know how mM varies with mL (because I
need that to analyze conformational
equilibria) The answer is
Stockmayer, 1950
Captures binding AND exclusion! Model-free
13
14
Binding to macromolecules
Now consider site-specific binding as model
Connect (also) this p.f. and m
14
15
Binding to macromolecules and conformation change
Equilibrium between two conformations, P and Q
depends on aL and Dn
15
16
Binding
Modified Grand Canonical Ensemble N systems
having the same V and able to exchange energy
and molecules ?, V, T ensemble Except that one
species (M) is not exchangeable
16
17
Binding
canonicalensemble
grand(canonical)ensemble
17
18
Binding
modified grand(canonical)ensemble
18
19
Binding
Modified GC p.f.
Binding without binding sites Also deals with
exclusion!
19
20
Binding
Relation to mass action formulation
20
21
Binding
Modified GC p.f.
The free energy of binding
21
22
Binding
Number of bound ligands and GC p.f.
can be determined experimentally
22