Building A Toolset - PowerPoint PPT Presentation
1 / 97
Title:
Building A Toolset
Description:
Use Conjugated Molecules; Carbonyl Group, Nitro Group ... The compound does not contain 'Carbonyl' or 'Nitro' groups. ... Double Bonds, Triple Bonds and Nitro Groups ... – PowerPoint PPT presentation
Number of Views:153
Avg rating:3.0/5.0
Title: Building A Toolset
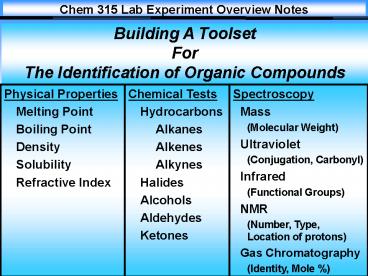
1
Building A Toolset For The Identification of
Organic Compounds
2
Spectroscopy
- Study of the Interaction of Electromagnetic
Radiation (Energy) and Matter - When energy is applied to matter it can be
absorbed, emitted, cause a chemical change
(reaction), or be transmitted. - Electromagnetic Spectrum
Cosmic ? (Gamma) X-Ray Ultraviolet Visible Infr
ared Microwave Radio
3
- Spectroscopy Types
- Mass Spectrometry (MS) Hi-Energy Electron
Bombardment - Use Molecular Weight, Presence of Nitrogen,
Halogens - Ultraviolet Spectroscopy (UV) Electronic Energy
States - Use Conjugated Molecules Carbonyl Group, Nitro
Group - Infrared Spectroscopy (IR) Vibrational Energy
States - Use Functional Groups Compound Structure
- Nuclear Magnetic Resonance (NMR) Nuclear Spin
States - Use The number, type, and relative position of
protons (Hydrogen nuclei) and Carbon-13
nuclei
4
- Mass Spectrometry
- High energy electrons bombard organic molecules
producing positive ions with a charge of 1
(cation radicals). - These ions are accelerated in an electric field
and separated according to mass-to-charge (M/z)
ratio in a magnetic field. - Since the charge on the ion is 1, Mass
Spectrometry provides information about the
Molecular Weight of a compound. - The mass spectrum produced is a plot of relative
abundance versus the Mass / Charge (M/Z) ratio. - The most intense peak is called the Base Peak,
which is arbitrarily set to 100 abundance all
other peaks are reported as percentages of
abundance relative to the Base Peak
5
- Mass Spectrometry (Cont)
- The Molecular Ion peak has the highest M/z in the
spectrum, i.e., the last peak on the right side
of the spectrum - The Molecular Ion Peak(s) abundance, i.e., peak
height, can be quite small. - The Molecular Ion peak represents the molecular
weight of the compound. - Molecular Ion peak is usually not the base peak!
- The Integral Molecular Weights reflect the
naturally occurring isotopic mixture of the
compound.
6
Mass Spectrometry (Cont)
Typical Mass Spectrum
M - (H2O and CH2 CH2)
1-Pentanol - MW 88CH3(CH2)3 CH2OH
Base Peak
M - (H2O and CH3)
M - H2O
CH2OH
Molecular Ion Peak (88)
7
Mass Spectrometry (Cont)
The Presence of Nitrogen in the Compound If the
Mass / Charge (m/z) ratio for the Molecular Ion
peak is Odd, then the molecule contains an Odd
number of Nitrogen atoms, i.e., 1, 3, 5,
etc. Note An Even value for the Mass / Charge
ratio could represent a compound with an even
number of Nitrogen atoms, i.e., 0, 2, 4 etc., but
the actual presence of Nitrogen in the compound
is not explicitly indicated as it is with an
Odd value for the ratio.
8
Mass Spectrometry (Cont) Most elements exist in
several isotopic forms Ex. 1H1, 2H1, 12C6, 13C6,
35Cl17, 37Cl17, 79Br35, 81Br35 Average Molecular
Weight The average molecular weight of All
isotopes of a given element relative to the
abundance of the each isotope in
nature. Integral Molecular Weight The Number
of Protons and Neutrons in a specific
isotope Each fragment represented in a Mass
Spectrum produces several peaks each representing
a particular isotopic mixture of the elements in
the compound.
9
- Mass Spectrometry (Cont)
- The Presence of Chlorine in a Compound
- The two (2) principal Chlorine Isotopes in nature
areCl-35 and Cl-37 (2 additional Neutrons in
Cl-37) - The relative abundance ratio of Cl-35 to Cl-37 is
100 32.6 or 75.8 24.2 or ?
3 1 - Therefore, a Molecule containing a single
Chlorine atom will show two Mass Spectrum
Molecular Ion peaks, one for Cl-35 (M) and one
for Cl-37 (M 2) - Note M 2 denotes 2 more neutrons than M
- Based on the natural abundance ratio of 100 /
32.6 (about 31), the relative intensity (peak
height) of the Cl-35 peak will be 3 times the
intensity of the Cl-37 peak.
10
1-Chloropropane
11
- Mass Spectrometry (Cont)
- The Presence of Bromine in a Compound
- The two (2) principal Bromine Isotopes in nature
areBr-79 and Br-81 (2 additional Neutrons in
Br-81) - The relative abundance ratio of Br-79 to Br-81 is
100 97.1 or 50.5 49.5 or ? 1
1 - Molecules containing a single Bromine atom will
also show two molecular ion peaks one for Br-79
(M) and one for Br-81 M2). - Based on the natural abundance ratio of 100 /
97.1 (about 11), the relative intensity of the
Br-79 peak will be about the same as the Br-81
peak.
12
- Mass Spectrometry Summary
- Fragmentation of Organic Molecules by high energy
electrons - Base Peak 100 abundance
- Molecular Ion Peak Highest Mass/Charge ratio
- Molecular Ion Peak Last peak(s) on right side
of chart - Molecular Ion Peak Represents Molecular Weight
of compound - Molecular Ion Peak If value is Odd the
compound contains an odd number of Nitrogen
atoms - Molecular Ion Peak If two peaks occur and the
relative abundance ratio is 31, then the
compound contains a single Chlorine atom. - Molecular Ion Peak If two peaks occur and the
relative abundance ration is 11, then the
compound contains a single Bromine Atom.
13
- Ultraviolet Spectroscopy (Cont)
- UV Spectroscopy is generally limited to the
determination of the presence of - Conjugated Unsaturated systems
- Carbonyl (CO) Groups.
- Nitro (NO2) Groups
- Conjugated Unsaturated Systems
- Conjugated unsaturated systems are molecules with
two or more double or triple (?) bonds each
alternating with a sigma (?) bond.
Ex. CH2CH ? CHCH2 - Conjugated systems absorb strongly in the UV /
Visible portion of the electromagnetic spectrum,
i.e., wavelengths longer than 200 nm therefore
they can be investigated with Ultraviolet
Spectroscopy.
14
- Practical Approach to Interpreting UV/Vis
Information - If the problem you are working on provides an
UV/Vis spectrum and it indicates No absorption
in the 200 700 nm range, the following
conclusions are applicable - The compound is not conjugated, i.e., it does not
contain alternating double/single bonds
(including Benzene ring.) - The compound does not contain Carbonyl or
Nitro groups. - If the problem provides Log Absorptivity values
(Log ?) the following possibilities exist - Log ? (gt 4.0) - Conjugated ?,? -
Unsaturated ketones,
Dienes,
Polyenes - Log ? (3.0 4.0) - Aromatic ring
(Check IR, NMR) - Log ? (1.5 2.2) - CO
(Check IR) - Log ? (1.0 1.5) - NO2
(Check IR)
15
Ultraviolet Spectroscopy Wavelength of Maximum
Absorbance ?max 230 nm
Molar Absorptivity ?
15,000 cm-1 Log ? 4.2
? Conjugated Molecule
(Benzene Ring)
16
Infrared Spectroscopy (IR) Infrared
Radiation That part of the electromagnetic
spectrum between the visible and microwave
regions 0.8 ?m (12,500 cm-1) to 50 ?m (200
cm-1). Area of Interest in Infrared
Spectroscopy The Vibrational portion of infrared
spectrum 2.5 ?m (4,000 cm-1) to 25 ?m (400
cm-1) Radiation in the Vibrational Infrared
region is expressed in units called wavenumbers (
) Wavenumbers are expressed in units of
reciprocal centimeters (cm-1) i.e. the reciprocal
of the wavelength (?) expressed in centimeters.
(cm-1) 1 / ?
(cm)
17
- Infrared Spectroscopy (IR)
- Molecular Vibrations
- Absorption of infrared radiation corresponds to
energy changes on the order of 8-40 KJ/mole (2-10
Kcal/mole - The frequencies in this energy range correspond
to the stretching and bending frequencies of the
covalent bonds with dipole moments. - Stretching (requires more energy than bending)
- Symmetrical
- Asymmetrical
- Bending
- Scissoring (in-plane bending)
- Rocking (in-plane bending)
- Wagging (out-of-plane bending)
- Twisting (out of plane bending)
18
- Infrared Spectroscopy (IR)
- No two molecules of different structure will have
exactly the same natural frequency of vibration,
each will have a unique infrared absorption
pattern or spectrum. - Two Uses
- IR can be used to distinguish one compound from
another. - Absorption of IR energy by organic compounds will
occur in a manner characteristic of the types of
bonds and atoms in the functional groups present
in the compound thus, infrared spectrum gives
structural information about a molecule. - The absorptions of each type of bond (NH, CH,
OH, CX, CO, CO, CC, CC, CC, CN, etc.) are
regularly found only in certain small portions of
the vibrational infrared region, greatly
enhancing analysis possibilities.
19
Infrared Spectroscopy (IR) The Infrared
Spectrum A plot of absorption intensity (
Transmittance) on the y-axis vs. frequency
(wavenumbers) on the x-axis.
CH
-C-H Satn
CH3
CO
20
Infrared Spectroscopy (IR) Principal Frequency
Bands (from left to right in spectrum) O-H 3600
cm-1 (Acids - Very Broad, Alcohols -
Broad) N-H 3300 - 3500 cm-1 (2, 1, 0 peaks 1o,
2o, 3o) CN 2250 cm-1 (Nitrile) CC 2150 cm-1
(Acetylene) CO 1685 - 1725 cm-1 (1715)
(Carbonyl) CC 1650 cm-1 (Alkene) 4 absorptions
1450-1600 (aromatic) CH2 1450 cm-1 (Methylene
Group) CH3 1375 cm-1 (Methyl Group) C-O 900 -
1100 cm-1 (Alcohol, Acid, Ester, Ether,
Anhydride) -C-H (Saturated Alkane absorptions
on Right side of 3000 cm-1) C-H (Unsaturated
Alkene absorptions on Left side of 3000
cm-1) C-H (Aromatic absorptions) Verify at
1667 2000 cm-1 C-H (Unsaturated Alkyne
absorptions on Left side of 3000 cm-1)
21
- Infrared Spectroscopy (IR)
- Suggested approach for analyzing IR Spectra
- Step 1. Check for the presence of the Carbonyl
group (CO) at 1715 cm-1. If
molecule is conjugated, the strong (CO)
absorption - will be shifted to the right by
30 cm-1,i.e., 1685 cm-1 - If the Carbonyl absorption is present, check
for - Carboxylic Acids - Check for OH group (broad
absorption near 3300-2500 cm-1) - Amides - Check for NH group (1 or 2
absorptions near 3500 cm-1) - Esters - Check for 2 C-O groups (medium
absorptions near 1300-1000 cm-1) - Anhydrides - Check for 2 CO absorptions near
1810 and 1760 cm-1 - Aldehydes - Check for Aldehyde CH group (2 weak
absorptions near 2850 and 2750 cm-1) - Ketones - Ketones (The above groups have been
eliminated)
22
Methyl IsoButyl Ketone (108-10-1)
IR SpectrumKetones
CH2
Bend
Saturated-C-H
Bend
CH3
CO Carbonyl
Bend
Mol Wgt 100.16 C 72.0 H 12.0 O 16.0
C6H12O
23
Nonanal
IR SpectrumAldehydes
CO Overtone
CH3
Aldehyde Hydrogen Stretch 2 Peaks
CH2
CO Carbonyl
Aliphatic C-H Stretch
C9H18O
CAS 124-19-6
24
Vinyl Acetate (108-05-4)
IR SpectrumEsters
Unsaturated C-H
CH3
Note 2 C-O Stretch Absorptions For Ester
Unsaturated C-H (alkene)
C-O
CO Carbonyl
C-O
Mol Wgt 86.09 C- 55.8 H- 7.0 O- 37.2
C4H6O2
25
Acetanilide (N-Phenylacetamide)
Mol Ion Peak Odd (Nitrogen Present)
Mol Ion Peak 135
26
Acetanilide (N-Phenylacetamide)
IR SpectrumAmides
C8H9NO
CAS 103-84-4
27
Benzamide
IR SpectrumAmides
Aromatic Overtones
UnsatdC-H Stretch
N-H Bend
OOP Bending Aromatic Monosubstitution
NH2 Stretch2 peaks Primary Amino
CO Carbonyl
CC Aromatic
Satd -C-H Stretch
C7H7NO
CAS 55-21-0
28
- Infrared Spectroscopy (IR)
- Step 2. - If the Carbonyl Group is Absent Check
for Alcohols, Amines, or
Ethers. - Alcohols Phenols - Check for OH group (Broad
absorption near 3600-3300 cm-1 Confirm
present of C?O near 1300-1000 cm-1 - Amines - Check for NH stretch (Medium
absorptions) near 3500 cm-1 - Primary Amine - 2 Peaks
- Secondary Amine - 1 Peak
- Tertiary Amine - No peaks
- N-H Scissoring at 1560 - 1640 cm-1
- N-H Bend at 800 cm-1
- Ethers - Check for C-O group near 1300- 1000
cm-1 and absence of OH
29
IR SpectrumAlcohols
2-Butanol (78-92-2)
C-O
CH3
CH2
-C-H Satn
OH
Mol Wgt 74.12 C- 64.8 H- 13.6 O- 21.6
C4H10O
30
Mass SpectrumAmines
P-Methoxybenzylamine (2393-23-9) (Primary Amine)
Molecular Ion Peak M/z - 137
C 70 H 8.1 C 0.70 137.18
96.03/12 8 H 0.081 137.18 11.1/1
11 137.18 (96 11.1) 30.08 O 16 N
14 16 14 30 ? C8H11NO
Mol Wgt 137.18 C- 70.0 H- 8.1 N- 10.2 O-
11.7
C8H11NO
31
IR SpectrumAmines
P-Methoxybenzylamine (2393-23-9) (Primary Amine)
D
G
A
B
C
F
J
I
E
H
Mol Wgt 137.18 C- 70.0 H- 8.1 N- 10.2 O-
11.7
C8H11NO
32
IR SpectrumAmines
P-Methoxybenzylamine (2393-23-9 (Primary Amine)
Aromatic Overtones P-Disubstitution
PrimaryAmine 2 Peaks
CH3
Unsaturated C-H
Saturated-C-H
CH2
OOP Bending P-Disubstitution
C-O
AromaticCC
C-O
Mol Wgt 137.18 C-70.0 H-8.1 N-10.2 O-11.7
C8H11NO
33
Phenetole (Unbalanced Ether)
IR SpectrumEthers
AromaticOvertones
Aliphatic C-H Stretch
Unsat C-H Stretch
CH3
C-O
Note 2 CO Bend Absorptions For Unbalance Ether
CH2
OOP Bending AromaticMonosubstitution
Aromatic ring CC Absorptions
C-O
C8H10O
CAS 103-73-1
34
- Infrared Spectroscopy (IR)
- Step 3. Refine the Structure Possibilities by
Looking for Double Bonds,
Triple Bonds and Nitro Groups - Double Bonds - Unsaturated CC (and CC) stretch
show absorptions on the left side of 3000
cm-1 - Alkene CC 2 weak absorptions near 1650 cm-1
- Aromatic CC (4 absorptions 1450-1650 cm-1)
- (Verify Aromatic at 1667 2000 cm-1)
- Triple Bonds - R-C N Nitrile - medium, sharp
absorption (stretch) near 2250 cm-1 R
C C R Alkyne - weak, sharp absorption
(stretch near 2150
cm-1) R C C H
Terminal Acetylene
(stretch near 3300 cm-1) - Nitro Groups - Two strong absorptions 1600 1500
cm-1 and 1390 - 1300 cm-1
35
Propargyl Alcohol (2-Propyn-1-ol)
IR SpectrumAlkynes (C?C)
CCStretch
Aliphatic C-H Stretch
OH H - Bonded
CH2
C-H Terminal Alkyne Stretch
C-O
C3H4O
CAS 107-19-7
36
- Infrared Spectroscopy (IR)
- Step 3 (Cont)
- Aromatic Ring Absorptions
- Aromatic unsaturated CC bonds show an absorption
on the left side of 3000 cm-1, but the
aromaticity must be verified in the overtone
region (1667 2000 cm-1) and the out-of-plane
(OOP) region (900 - 690 cm-1) - 4 Medium to strong absorptions in region 1650 -
1450 cm-1 - Many weak combination and overtone absorptions
appear between 2000 and 1667 cm-1 - The relative shapes and numbers (1 - 4) of the
overtone absorptions can be used to tell whether
the aromatic ring is monosubstituted or di-,
tri-, tetra-, penta-, or hexa-substituted. - Positional (ortho (o), meta (m), para (p))
isomers can also be distinguished. - Note A strong carbonyl absorption can overlap
these overtone bands, making them unusable.
37
- Infrared Spectroscopy (IR)
- Step 3 (Cont)
- Aromatic Ring Absorptions (Cont)
- The unsaturated C-H Out-of-Plane (OOP) bending
absorptions in the region 900 690 cm-1 can also
be used to determine the type of ring
substitution. - The number of absorptions and their relative
positions are unique to each type of
substitution. - Although these absorptions are in the
Fingerprint region they are particularly
reliable for rings with Alkyl group
substitutions. - They are less reliable for Polar substituents.
38
Mass SpectrumNitriles
Benzonitrile (100 - 47- 0)
Molecular Ion Peak M/z - 103
Mol Wgt 103.12 C- 81.5 H- 4.9 N- 13.6
C7H5N
39
(No Transcript)
40
IR SpectrumNitriles
Benzonitrile (100 - 47- 0)
Aromatic Overtones Monosubstitution
Unsaturated C-H Stretch
Aromatic OOP Mono Substitution
Nitrile -CN
Aromatic CC
Mol Wgt 103.12 C- 81.5 H- 4.9 N- 13.6
C7H5N
41
Infrared Spectroscopy (IR) Step 4. If none of the
above apply then the compound is most likely
a Hydrocarbon Alkyl Halide Generally, a very
simple spectrum Hydrocarbons -C-H Alkane Satn
(gt 3000 CH2 (1450 cm-1) CH3 (1375 cm-1)
42
N-Hexylbenzene (1077-16-3)
IR SpectrumAlkane / Aromatic Ring
Aromatic Overtones Mono Substitution
CH3
C-HAromatic
OOP Bending Mono Substitution
CH2
Alkene(CC)Aromatic(CC)Stretch
Aliphatic Alkane -CH3, -CH2-Stretch
Mol Wgt 162.27 C-88.9 H-11.1
C12H18
43
IR Spectrum Halides
3-Bromo-1-propene (106-95-6)
Saturated-C-H
Unsaturated C-H (alkene)
Unsaturated C-H
CH2
CH2-Br
Br
Mol Wgt 120.98 C - 29.8 H - 4.1 Br - 66.1
C3H5Br
44
Carbonyl (CO) _at_ 1715-1685 (Conjugation moves
absorption to right 30 cm-1
Yes
No
Infrared Spectroscopy (IR)
Acid Ester Amide Anhydride Aldehyde Ketone
Alcohol Amine Ether
Saturation lt 3000 cm-1
Unsaturation gt 3000 cm-1
Alkenes (Vinyl) -CC Alkynes (Acetylenes) -CC Aro
matic (aryl) -CC
Alkanes -C-H Methylene -CH2 Methyl -CH3
Alkyl Halides
Nitriles
Nitro
Hydrocarbons
45
- Melting Point
- Temperature at which a transition occurs between
solid and liquid phases - Temperature at which an equilibrium exists
between the well-ordered crystalline state and
the more random liquid state.
- Melting Point Range
- The first point (lower temperature) is the
temperature at which the first drop of liquid
forms amongst the crystals. - The second point (higher temperature) is the
temperature at which the entire mass of solid
turns to a clear liquid.
- Uses
- Identify Compounds
- Establish Purity of Compounds
46
- Melting Point
- Melting Point Indicates Purity in Two Ways
- The Purer the Compound, the Higher the Melting
Point - The Purer the Compound, the Narrower the Melting
Point Range
- Melting point of A decreases as impurity B is
added - Eutectic Point is the Solubility Limit of B in A
Thus, it is the Lowest Melting Point of an A/B
mixture(Note Sharp melting point no range at
eutectic point)
47
- Refractive Index
- Refractive Index - A physical property of
condensed liquids solids - Uses
- Identification
- Measure of Purity
- Definition
- Light travels at different velocities in
condensed phases (liquids, solids) than in air. - Light travels more slowly through a denser
substance. - The wavelength of light is also different in
condensed phases. - As the velocity decreases, the wavelength
decreases. - The frequency of light in condensed phases does
not change.
48
- Refractive Index
- The Refractive Index for a given medium depends
on two (2) variables - Refractive Index (n) is wavelength (?)
dependent.Beams of light with different
wavelengths are refracted to different extents in
the same medium, thus, produce different
refractive indices. - Refractive Index (n) is temperature dependent.As
the temperature changes, the density changes
thus the velocity (?) changes. - Density of a medium decreases as temperature
rises. - Speed of light in medium increases as temperature
rises and density decreases. - Ratio of speed of light in vacuum vs. speed of
light in medium decreases, thus, the Refractive
Index decreases as temperature rises.
49
- Refractive Index - Reading the Instrument
- Index of Refraction (ND) decreases with
increasing temperature, i.e., velocity of light
in medium increases as density decreases. - Measured values of (ND) are adjusted to 20oC
For temp gt 20oC (?t is positive), i.e., add
correction factor For temp lt 20oC (?t is
negative), i.e., subtract correction factor - Temp Correction Factor ?t 0.00045 (Room
Temp 20) 0.00045 - The following equation automatically accounts for
temp correction ND20
NDRm Temp (Rm Temp 20) 0.00045 - Ex For an observed value of 1.5523 at 16oC,
the correction is - ND20 1.5523 (16 20) 0.00045
1.5523 (-4) 0.00045 - Note Instrument can be read
to 4 decimal places
- Typical Range of Values for Organic Liquids
1.3400 - 1.5600
50
- Recrystallization
- Solid organic compounds produced in the
laboratory usually need to be purified. - The most common technique involves
Recrystallizing the sample. - The Recrystallization process is a relatively
slow and selective formation of crystals from a
solvent. - Precipitation is a rapid and nonselective
process thus not used to purify samples.
51
- Recrystallization (Cont)
- General Process
- Dissolve sample in a minimal amount of an
appropriate solvent. - Sample should be insoluble in solvent at room
temperature, but soluble at elevated (boiling
point) temperature. - If solution is colorized, it is sometimes
necessary to add a decolorizing agent (activated
charcoal - Norite) - Colorized solutions are first filtered through a
fluted filter or a column containing alumina or
silica gel.
52
- Recrystallization (Cont)
- General Process (Cont)
- The hot solution is cooled slowly to room
temperature.As Temperature changes the solute
particles begin to come out of solution, leaving
the more soluble impurities in solution. - After crystallization, place beaker in water/ice
bath. - Collect crystals by vacuum filtration.
- Rinse the crystals with small portion of cold
solvent. - Dry the crystals in air in your drawer.
- Determine Melting Point of dried sample.
53
- Recrystallization (Cont)
- The Appropriate Solvent
- The solute particles are generally insoluble in
cold solvent, but soluble in hot solvent. - The solvent (or mixed solvent) should have a
steep solubility vs temperature curve.
- The C curve is a good solvent
- Solute sparingly soluble at room temperature
- Solute very soluble at elevated temperature
54
- Recrystallization (Cont)
- The Appropriate Solvent (Cont)
- Solubility of organic compounds is a function of
the polarities of both the solvent and the
solute - Like Dissolves Like
- Polar solvents dissolve polar solutes
- Nonpolar solvents dissolve nonpolar solutes
- The stability of the solute crystal lattice
affects the solubility. The higher the melting
point (higher stability), the less soluble the
solute.
55
- Recrystallization (Cont)
- The appropriate Solvent (Cont)
- The Boiling Point of the solvent must be less
than the Melting Point of the solute.If the
Boiling Point of the solvent is higher than the
melting point of the solute, the solute will
Melt instead of Dissolving in the solvent at
the elevated temperature.Upon cooling, the
Melted solute will Oil out forming an
insoluble mass that is not purified - The solvent should not react with the solute
- Compounds with functional groups that can form
hydrogen bonds (-OH, -NH-, -COOH, -CONH-) will be
more soluble in hydroxylic (polar) solvents such
as Methanol and Water.
56
- Filtration
- Two Purposes
- Separate Purified Solid from the Soluble
Impurities in the Solution from which it was
Recrystallized. - Remove Solid Impurities from a Liquid
- Two Types
- Gravity
- Vacuum
- Filter Paper
- Retentivity Opposite of Porosity measure of
the size of particles that can be retained on
the filter paper. - Porosity Measure of the size of the particles
than can pass through the paper.
57
- Gravity Filtration Types
- Filter Cones - Folded paper filter inserted into
a class funnel with stem extending into a
receiving flask. Applicable Volume gt 10 mL. - Fluted Filters - Specially folded (many creases)
filter paper inserted into a class funnel
with stem extending into a receiving
flask. Applicable Volume gt 10 mL
58
- Gravity Filtration Types (Cont)
- Filtering Pipettes - Microscale technique used
with Pasteur Pipets. A piece of cotton is
inserted into the top of the lower
constriction. Applicable Volume lt 10mL - Decantation - Careful pouring of supernatant
liquid into another vessel leaving solids
particles behind.
59
- Vacuum Filtration - More rapid than gravity.
- Buchner Funnels - Primarily used to filter large
volumes of liquid from solids, such as
crystals from the Recrystallization
Process. Applicable Volume gt 10 mL. - Hirsch Funnels - Similar, but smaller than
Buchner Funnel, with sloping sides. Used in
Microscale techniques. - Applicable Volume lt 10 mL.
60
- Simple Fractional Distillation
- Distillation Process of vaporizing a liquid,
condensing the vapor, and collecting the
condensate in another container. - Uses
- Separating liquids with different boiling points
- Purifying a liquid.
- Methods
- Simple
- Vacuum (at reduced pressure)
- Fractional
- Steam
61
Simple Fractional Distillation Pure
Substance Temperature remains constant during
distillation process so long as both vapor and
liquid are present Liquid Mixture Temperature
increases throughout process because composition
of vapor changes continuously. Composition of
vapor in equilibrium with the heated solution is
different from the composition of the solution.
62
Simple Distillation Single vaporization-
condensation cycle of a mixture that produces a
distillate that is always impure at any
temperature range between the range of boiling
points of the components. Therefore, it is
impossible to completely separate the components
in a mixture with simple distillation. Relatively
pure substances can be obtained from a mixture
with simple distillation if the boiling points of
the components differ by a large amount
(gt100oC) Distilling the distillate from multiple
sequential vaporization-condensation cycles
would produce increasingly pure substances, but
this is a very tedious process.
63
Fractional Distillation Accomplishes the same
thing as multiple simple sequential
vaporization-condensation cycles, by inserting a
fractionating column between the distillation
flask and the distilling head. With each
Vaporization-Condensation Cycle within the
column, the composition of the vapor is
progressively enriched in the lower boiling
liquid. When the lower boiling liquid is
effectively removed from the original mixture,
the temperature rises and a second fraction
containing some of both compounds is produced. As
the temperature approaches the boiling point of
the higher boiling point compound, the distillate
condensing into the third receiving flask is
increasingly pure in the higher boiling point
compound.
64
- Simple Fractional Distillation
Procedures/Computations - Physical Characteristics of Standard Mixture
- Solubility Density Relative to Water
- Distillation (Simple Fractional in one
Procedure) - Compute Total Volume Recovered
- Compute Percent () Recovery
- Plot Bar Chart of Temperature Increments (X-axis)
vs. Volume Increments (Y-axis) - Draw Perpendicular Line to 105oC mark on X-axis
- Compute Fractional Volumes of Ethyl Acetate
(Increments to left of line) and Butyl Acetate
(Right of Line) - Compute Volume Percent () Recovery of Ethyl
Fraction and Butyl Fraction
65
- Simple Fractional Distillation
Procedures/Computations - Compute Mass of Ethyl Acetate and Butyl Acetate
from Fraction Volumes and Density - Compute Moles of Ethyl Acetate and Butyl Acetate
- Compute Total Moles
- Compute Mole Fractions of Ethyl Acetate and Butyl
Acetate - Compute Mole Percents of Ethyl Acetate and Butyl
Acetate
66
- Gas Chromatography
- Uses
- Separation and analysis of organic compounds
- Testing purity of compounds
- Determine relative amounts of components in
mixture - Compound identification
- Isolation of pure compounds (microscale work)
- Similar to column chromatography, but differs in
3 ways - Partitioning process carried out between Moving
Gas Phase and Stationary Liquid Phase - Temperature of gas can be controlled
- Concentration of compound in gas phase is a
function of the vapor pressure only.
67
- Gas Chromatography
- Principals of Separation
- Column is selected, packed with Liquid Phase, and
installed. - Sample injected with microliter syringe into the
injection port where it is mixed into the carrier
gas stream (helium, nitrogen, argon). - Sample becomes partitioned between Moving Gas
Phase and Stationary Liquid Phase. - The time the different compounds in the sample
spend in the Vapor Phase is a function of their
Vapor Pressure (related to boiling point
molecular weight). - The more volatile (Low Boiling Point / Higher
Vapor Pressure) compounds arrive at the end of
the column first and pass into the detector
68
- Gas Chromatography
- Factors Affecting Separation
- Boiling Points of Components in Sample
- Low boiling point compounds have higher vapor
pressures. - High boiling point compounds have lower vapor
pressures requiring more energy to reach
equilibrium vapor pressure, i.e., atmospheric
pressure. - Boiling point increases as molecular weight
increases. - Flow Rate of Carrier Gas
- Choice of Liquid Phase
- Molecular weights, functional groups, and
polarities of component molecules are factors in
selecting liquid phase. - Length of Column
- Similar compounds require longer columns than
dissimilar compounds. Isomeric mixtures often
require quite long columns
69
- Gas Chromatography
- Retention Time
- The period following injection that is required
for a compound to pass through the column to the
point where the detector current is maximum, i.e.
maximum pen deflection or maximum peak height. - For a given set of constant conditions (carrier
gas, flow rate of carrier gas, column
temperature, column length, liquid phase,
injection port temperature), the retention time
of any compound is always constant. - Retention Time is similar to the Retardation
Factor, Rf in Thin Layer Chromatography. - Compute Retention Time from the Chart Speed (5
cm/min) and the distance on the chart from the
time of injection to the point on the chart where
the perpendicular line drawn from the peak height
intersects the base line.
70
Gas Chromatography Peak Area Mole Percent
Relationship The area under a gas chromatograph
peak is proportional to the amount (moles) of
compound eluted. The molar percentage composition
of a mixture can be approximated by comparing the
relative areas of the peaks in the
chromatogram. This method assumes that the
detector is equally sensitive to all compounds
and its response is linear. Triangulation Method
of Determining Area Under Peak. Multiply the
height of peak (in mm) above the baseline by the
width of the peak at half the height.
Baseline is a straight line connecting side arms
of peak. Best if peaks are
symmetrical. Add areas to get total area. Divide
each area by total area to get mole fraction.
71
- Gas Chromatography of Acetates Procedures /
Calculations - Obtain Gas Chromatograph of a Mixture of 4 Known
Acetates. - Obtain Gas Chromatograph of a Mixture of 1 to 4
Unknown Acetates - Compute the Retention Times of Each Peak
- Calculate the Area of Each Peak Using the
Triangulation Method - Compute the Total Area of the Peak on the
Chromatogram - Compute the Mole Fraction of Each Peak from the
Peak Areas - Compute the Mole Percent of Each Peak
- Compare the Retention Times of the Unknown Peaks
with the Known Mixture and Determine the Number
and Identity of Compounds in the Unknown Mixture.
72
- Synthesis of t-Butyl Chloride
- Reaction of t-Butyl Alcohol (or t-Pentyl Alcohol)
with conc. HCL to form t-Butyl Chloride (or
t-Pentyl Chloride). - Three-step Sn1 Nucleophilic Substitution
Reaction. - This is a First Order Rate Reaction where the
Rate of Formation of t-Butyl Chloride (t-Pentyl
Chloride) is dependent only on the concentration
of the Alcohol it is independent of the amount
of acid (HCL) used. - NOTE Rate of Formation and Limiting Reagent are
independent of each other. Thus, Limiting Reagent
must be computed. - Stoichiometric Balanced Equation
73
- Synthesis of t-Butyl Chloride
- Elements of the Experiment
- Determining the masses of the reactants (Alcohol,
HCl) - Determining the moles of the reactants
- Setting up the Stoichiometric equation
- Determining the Limiting Reagent
- Determining the Theoretical Yield
- Mixing reagents and initiating the reaction
- Separating Washing the product (H2O, NaHCO3)
- Drying the Product
- Purifying B.Pnt of the Product by Simple
Distillation - Drying the Product again
- Determining the Mass (Yield) of the Product
- Determining the Refractive Index
- Obtaining the Infrared Spectrum
74
- Synthesis of t-Butyl Chloride
- Determination of the Limiting Reagent /
Theoretical Yield - The Experimental Yield (mass or moles) of any
synthesized compound is compared to the
Theoretical Yield of product, i.e., the yield
is computed. - The Theoretical Yield is determined from the
following - The Mass, in Moles, of the reagents actually used
in the experiment. - Stoichiometric Balanced Equation
Ex. A B ? C D - Molar Ratio (1 1 1 1 in above
example) - Limiting Reagent
- Molecular Weight of Product
75
- Synthesis of t-Butyl Chloride
- The Molar Ratio is obtained from the balanced
equation, i.e., the number of moles of reagent
A that will react with a molar equivalent
number of moles of reagent B to produce a molar
equivalent number of moles of each product
111 121 231, 212, etc. - For t-Butyl Chloride the ratio is 1 1
1 1 - The ratio of moles of the reagents actually used
in the reaction is usually not the same as the
Molar Ratio from the equation. - The Limiting Reagent is that reactant, which is
totally consumed in the reaction leaving some of
the other reactant unreacted, i.e., the other
reactant is in excess. - The Limiting Reagent, thus, determines the
maximum amount of product (on a molar equivalent
basis) that can be produced in the reaction,
i.e., the Theoretical Yield.
76
- Synthesis of t-Butyl Chloride
- For the reaction between t-Butyl Alcohol and HCL
the masses are determined as follows - Determine the mass of the alcohol to the nearest
0.001 gram. - Determine the volume of the conc HCL solution to
the nearest 0.1 mL.Compute the mass of the HCL
from the volume and the density (see
table)Adjust the HCl mass value to account for
the composition of Conc HCl 37.3 - Calculate the Moles of each reagent by dividing
the Mass by its Molecular Weight.
moles mass / mol wgt.
77
- Synthesis of t-Butyl Chloride
- Note Moles of HCl can also be computed
directly
from the Volume and Molarity (12.0 moles/L) - From the balanced reaction equation determine the
molar ratio among the reactants and
productsi.e., How many moles of Alcohol react
with how many moles of HCL to give how many moles
of t-Butyl (t-Pentyl) Chloride. The ratio here is
1111 - If the ratio of moles of Alcohol to moles of HCl
actually used is greater than the reaction molar
equivalent ratio, then the Alcohol is in Excess
and HCl is Limiting. - If, however, the actual molar ratio is less than
the reaction molar ratio, then HCl is in excess
andt-Butyl (t-Pentyl) Alcohol is Limiting.
78
- Synthesis of t-Butyl Chloride
- Limiting Reagent Calculation Examples
- A B ? C
- Molar ratio AB 1 1 1.0
- Moles actually used A
0.05 B 0.12 - Molar ratio AB actually used 0.05 / 0.12
0.42 - Only 0.05 moles of the 0.12 moles of B would
be required to react with the 0.05 moles of
A available. - Since 0.05 lt 0.12
- B is in excess, A is
limiting.
79
- Synthesis of t-Butyl Chloride
- Limiting Reagent Calculation Examples (Cont)
- A 2B ? C
- Molar ratio AB 1 2 0.5
- Moles actually used A 0.0069 B
0.023 - Molar ratio AB actually used 0.0069 /
0.023 0.30 - Only 0.0069 ? 2 0.0138 moles of B are
required to react with 0.0069 moles of A. - Since 0.0138 lt 0.023
- B is in excess, A is limiting.
- Any actual molar ratio less than the reaction
molar ratio indicates B is in Excess and
A is Limiting. - Any actual molar ratio greater than the
reaction molar ratio indicates A is in
Excess and B is Limiting.
80
Synthesis of t-Butyl Chloride Limiting Reagent
Calculation Examples (Cont) In the
Friedel-Crafts alkylation of Biphenyl with
t-Butyl Chloride to form 4,4-Di-tert-Butyl
Biphenyl, 1.064 g of Biphenyl is reacted with
2.129 g of t-Butyl Chloride. The stoichiometric
equation indicates that 2 moles of t-Butyl
Chloride reacts with 1 mole of Biphenyl.
Determine the Limiting Reagent and the
Theoretical Yield.
In the above example, Biphenyl is the limiting
reagent because 0.0069 moles is less than 0.023 /
2 0.0115 moles. Thus, a maximum of 0.0069 moles
(1.838 g) 4,4di-tert-Butyl Biphenyl can be
expected.
81
- Qualitative Organic Analysis
- Organic compounds fall into classes alkanes,
alkenes, alcohols, esters, acids, nitriles,
ketones, aldehydes, etc. - These classes are effectively defined by the
functional group(s) associated with the
compounds. - The tests in the Qualitative Organic experiment
can be used to identify a select group of
classes. - Classes Compounds without a Carbonyl group or
nitrogen group. - Alkanes C-C
- Alkenes CC
- Alkynes C?C
- Aromatics CC
- Alkyl (1o, 2o, 3o) Aryl Halides R-X
- Alcohols (1o, 2o, 3o) R-OH
82
The Tests Compound Classes Test Compound
Class Solubility (H2O, H2SO4) All Beilstein
(Flame) Alkyl Aryl Halides Silver
Nitrate/Ethanol Alkyl Aryl Halides Sodium
Iodide/Acetone Alkyl Aryl Halides Bromine/Methyl
ene Chloride Unsaturated CC C?C KMnO4 (Baeyer
Test) Unsaturated CC C?C Ignition Aromaticity
CC Acetyl Chloride Alcohols Lucas
Test Alcohols Chromic Acid Alcohols
83
- Solubility Test(Water (H2O) and Conc Sulfuric
Acid (H2SO4) - Water Solubility
- Compounds with lt5 Carbons containing O, N, S are
soluble. - Compounds with 5-6 Carbons containing O, N, S are
borderline (slightly soluble). - Branching Alkyl chains result in lower
melting/boiling points and increased solubility. - Increase N, O, S to Carbon ratio increases
solubility.
84
Solubility Test(Water (H2O) and Conc Sulfuric
Acid (H2SO4)
- Conc H2SO4 Solubility
- Compounds containing N, O, S can be protonated in
Conc H2SO4 and thus are considered soluble. - Alkenes (CC)
- Alkynes (CC)
- Ethers (C-O-C)
- Nitroaromatics (Nitrobenzene)
- Amides
- Alcohols (R-OH)
- Ketones
- Aldehydes
- Esters
- Not soluble in Conc H2SO4 (Inert Compounds)
- Alkanes
- Aromatic Hydrocarbons
- Alkyl Halides
- Aromatic Halides
85
- Beilstein Test (General for Halides)
- Compound first burns with yellow flame.
- After burning for a few seconds, a green flame is
produced if a halogen is present. - Does not differentiate between chlorine, bromine,
or iodine. - Weak color could indicate present of impurities
in a non-halide sample.
86
- Silver Nitrate in Ethanol Test (Sn1 for
Halides) - Does not distinguish between Chloride, Bromine,
or Iodine. - Sn1 (nucleophilic substitution) reactions depend
on - Weak Nucleophile (NO3)
- Polar Solvent (Ethanol)
- Compounds equipped with good leaving groups (H2O,
CL, Br, I) - Compounds that can form stable carbocations
- Benzyl ? Allyl gt Tertiary (3o) gt Secondary
(2o) gt Primary (1o) gt Methyl gt Vinyl gt Aryl
(Aromatic).
87
- Silver Nitrate in Ethanol Test (Sn1 for
Halides) - The halide (the leaving group) is replaced with
the nitrate nucleophile. - The halide reacts with the Ag to form an
insoluble Silver Halide precipitate. - Positive test white precipitate insoluble in 5
HNO3 - Allyl, benzyl, tertiary halides give positive
test. - Primary secondary alkyl halides test positive
when heated (100oC). - Aromatic and many vinyl substituted halides do
not give positive tests.
88
- Sodium Iodide in Acetone (Sn2 for Alkyl
Halides) - Sodium Iodide is soluble in Acetone, but Sodium
Chloride and Sodium Bromide are not soluble. - The Iodide ion is an excellent Nucleophile - A
Lewis Base with a pair of unshared electrons that
seeks a positive part of an atom. - Acetone is a non-polar solvent.
- Alkyl Chlorides and Bromides would react with the
Sodium Iodide in a reaction to substitute the
Chloride Bromide ions with the Iodide ions. - As the Chloride Bromide ions are produced they
would precipitate. - Unreactive Chlorides Bromides (Benzyl and
Aromatic) would not produce a reaction, thus no
precipitates.
89
- Sodium Iodide in Acetone (Sn2 for Alkyl
Halides) - Relative Halide reactivity for an Sn2 reaction is
the opposite of an Sn1 reaction, that is - Vinyl gt Methyl gt Primary (1o) gt Secondary (2o) gt
Tertiary (3o) gt Allyl ? Benzyl ? Aryl
(Aromatic) - Note Aryl (Aromatic) Halides are unreactive
for both Sodium Iodide (Sn2) and Silver Nitrate
(Sn1) tests. - Primary Alkyl Halides will give an immediate
precipitate at room temperature. - Secondary Alkyl Halides will give a precipitate
when heated to 50oC and then cooled. - Tertiary Alkyl Halides will also give a
precipitate when heated to 50oC and then cooled. - Aryl Halides, like Chlorobenzene, will not give a
precipitate, even after heating.
90
- Bromine in Methylene Chloride (Simple Multiple
Bonds) - Addition of Bromine (Br2), a red liquid, to a
compound containing a double or triple bond
produces a colorless Dibromide. - The Double bond is converted through the addition
reaction to an aliphatic (alkane) structure - The double (or triple bond) must be sufficiently
electron-rich to initiate the reaction, i.e.,
minimal electron withdrawing groups attached to
molecule. Thus, the presence of carboxyl groups
would hinder the reaction.
91
- Potassium Permanganate (Baeyer) Test(double or
triple bonds) - Potassium Permanganate (KMNO4) is an oxidizing
agent. - It has a Purple color
- Following the oxidation of an unsaturated
compound, the Permanganate ion is reduced to
Manganese Dioxide (MnO2), a brown precipitate. - The test is positive for double or triple bonds,
but not aromatic rings.
92
- Potassium Permanganate (Baeyer) Test(double or
triple bonds) - Note Other easily oxidized compounds
aldehydes, some alcohols, phenols, and aromatic
amines should be accounted for in your analysis.
93
Ignition (Aromaticity) (C-H bonds in Aromatic
rings)
- The Test
- In a hood, place a small amount of the compound
on a spatula and place it in the flame of a
Bunsen burner. - Positive test is a sooty yellow flame.
- Note The Sooty flame usually comes off
fairly quickly. Look for it moving
quickly away and upward from the
yellow/blue flame area. - Positive test is indicative of a high degree of
unsaturation and is probably aromatic
94
- Acetyl Chloride (Alcohols)
- Acid Chlorides react with Alcohols to form
esters. - Acetyl Chloride forms Acetate esters.
- This test does not work well with solid alcohols.
- Phenols also react with Acetyl Chloride and
should be eliminated prior to testing for
Alcohols. - Amines also react with Acetyl Chloride to produce
heat and also should be eliminated prior to
testing. - The Test
- Positive test is evolution of Heat and
Hydrogen Chloride (HCl) gas.
95
- Lucas Test (Alcohols)
- Primary Alcohols
- Dissolve in reagent giving clear solution.
- Secondary Alcohols
- Cloudiness after about 3-5 minutes.
- May need to heat slightly.
- Tertiary, Benzylic, and