Fig 173 - PowerPoint PPT Presentation
1 / 44
Title: Fig 173
1
(No Transcript)
2
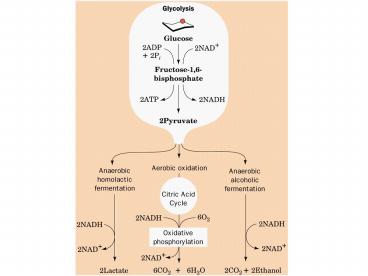
Fig 17-3
3
During study of pathways pay attention to
- 1) Changes in structures of intermediates (esp.
in carbon skeleton and in oxidation states of C
atoms). - 2) Phosphate group transfer.
- 3) Electron transfer
- (to/from NAD/NADH etc.)
4
TCA cycle ox. phosphorylation
5
Hexokinase reaction
- C6 hydroxyl group attacks g-phospate of ATP
(direct phosphate group transfer). - Logic
- ATP consumption increases glucose transport into
cell - G-6-P is first intermediate in several pathways
6
Phosphoglucose isomerase reaction
- 1,2 carbonyl shift via enediolate intermediate
- Logic
- This sets up the aldolase reaction to yield two
C3 species
7
(No Transcript)
8
(No Transcript)
9
Phosphofructokinase reaction
- Direct attack of 1 hydroxyl on g-phosphate of
ATP - Logic
- This is the committed step for the glycolytic
pathway (site of regulation and DG ltlt0) - The two C3 species generated in next step are
energetically equivalent because both halves of
the fructose are phophorylated.
10
Aldolase reaction
- Reverse Aldol results in C-C bond cleavage
between Ca and Cb (relative to carbonyl) - Logic
- Cleavage of C6 species to simpler compounds under
physiological conditions. - Products are easily interconverted thus, all
subsequent enzymatic steps are common to both C3
fragments.
11
(No Transcript)
12
(No Transcript)
13
Triose phosphate isomerase reaction
- 1,2 carbonyl shift via enediolate intermediate
- Logic
- Metabolic economy interconversion of GAP and
DHAP allows both C3 fragments of glucose to be
utilized by a single set of enzymes - Stereoelectronic control by TIM prevents
formation of toxic methyl glyoxal
14
(No Transcript)
15
(No Transcript)
16
Glycolytic pathway. First half energy
consuming glucose ? DHAP GAP Second half
energy producing 2GAP ? 2 pyruvate
17
GAPDH reaction
- Oxidation of CHO to -COOH with phosphoryl
transfer to yield a high energy mixed anhydride - Logic
- The major step in energy extraction
- Sets up a substrate-level phosphorylation and
generates NADH (even more ATP from e- transport
and oxidative phos.)
18
(No Transcript)
19
(No Transcript)
20
Table 16.3
21
Phospoglycerate kinase reaction
22
Phospoglycerate mutase reaction
- 1,2-Phosphoryl shift
- Logic
- Sets up the synthesis of pyruvate (i.e., an
a-keto acid- important for TPP reactions)
23
(No Transcript)
24
detour to make 2,3-BPG
25
What explains these curves?
26
Enolase reaction
- Elimination of water CC bond formation
- Logic
- Formation of a high-energy compound for substrate
level phosphorylation
27
(No Transcript)
28
Pyruvate kinase reaction
- Substrate-level phosphorylation
- Logic
- This is the pay off. The reaction generates
ATP in excess of initial investment
29
(No Transcript)
30
(No Transcript)
31
(No Transcript)
32
(No Transcript)
33
Lactate dehydrogenase (LDH)
NADH, H
NAD
lactate
34
The two steps of Alcoholic Fermenation
35
(No Transcript)
36
alcohol dehydrogenase (ADH)
37
(No Transcript)
38
(No Transcript)
39
TCA cycle ox. phosphorylation
40
(No Transcript)
41
T state (inactive) R state (active)
42
(No Transcript)
43
(No Transcript)
44
F-2,6-BP is the most potent allosteric activator
of PFK ATP is an inactivator of PFK