PO 1'637 - PowerPoint PPT Presentation
1 / 1
Title:
PO 1'637
Description:
Michael J. Ingleson - Royal Society University Research Fellow. michael. ... ( 3a) R. H. Crabtree et al., JACS, 1999, 121, Pg6337 (3b) I. Manners et al., Inorg. ... – PowerPoint PPT presentation
Number of Views:140
Avg rating:3.0/5.0
Title: PO 1'637
1
Michael J. Ingleson - Royal Society University
Research Fellow michael.ingleson_at_manchester.ac.uk
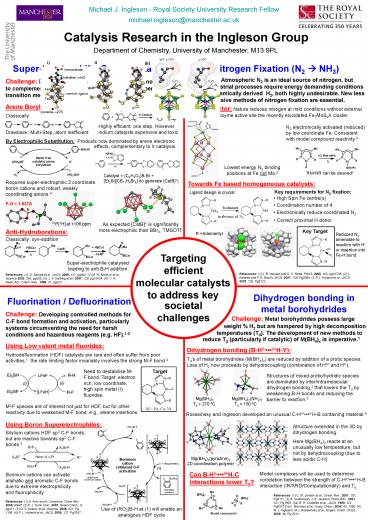
Catalysis Research in the Ingleson Group
Department of Chemistry, University of
Manchester, M13 9PL
Super-Electrophilic Organocatalysts
Nitrogen Fixation (N2 ? NH3)
Challenge Atmospheric N2 is an ideal source of
nitrogen, but current industrial processes
require energy demanding conditions and
petrochemically derived H2, both highly
undesirable. New less energy intensive methods of
nitrogen fixation are essential.
Challenge Development of super-electrophilic
organocatalysts to complement (and potentially
replace) toxic and expensive transition metal
catalysts.1
Arene Borylation
FeMo cofactor Nature reduces nitrogen at mild
conditions without external H2,1 with the enzyme
active site the recently elucidated Fe7MoS9X
cluster.
Catalytically2,3
Classically
Highly efficient, one step. However iridium
catalysts expensive and toxic
N2 electronically activated (reduced) by low
coordinate Fe. Consistent with model compound
reactivity.3
Drawback Multi-Step, atom inefficient
By Electrophilic Substitution
Products now dominated by arene electronic
effects, complementary to Ir catalysis.
Lowest energy N2 binding positions at Fe not Mo.2
RNNR can be cleaved!
Catalyst (C6H4O2)B-Br Et3SiCB11H6Br6 (to
generate CatB)
Requires super-electrophilic 2 coordinate boron
cations and robust, weakly coordinating anions.4
Towards Fe based homogeneous catalysts
Key requirements for N2 fixation
Ligand design is crucial
Si
- High Spin Fe centre(s)
- Coordination number of 4
- Electronically reduce coordinated N2
- Correct proximal H donor
P-O 1.637Å
Br
O
As expected CatB is significantly more
electrophilic than BBr3, TMSOTf,
31P1H at 106 ppm
Anti-Hydroborations
Key Target
R Adamantyl
Reduced N2 amenable to reaction with H, or
insertion into Fe-H bond
Classically syn-addition
Targeting efficient
Super-electrophile catalysed leading to anti B-H
addition
References (1) J. B. Howard and D. C. Rees,
PNAS, 2006, 103, pg10788. (2) J. Kastner and P.
E. Blochl, JACS, 2007, 129, Pg2998. (3) P.L.
Holland et al., JACS, 2007, 129, Pg8122.
References (1) D. Sames et al., JACS, 2009,
131, pg402. (2) M. R. Smith III et al., Science
2003, 295, pg305, (3). J. F. Hartwig et al.,
2007, 129, pg15434. (4). C. A. Reed, Acc. Chem.
Res., 1998, 31, pg325
molecular catalysts to address key
Dihydrogen bonding in metal borohydrides
Fluorination / Defluorination
societal challenges
Challenge Developing controlled methods for C-F
bond formation and activation, particularly
systems circumventing the need for harsh
conditions and hazardous reagents (e.g. HF).1-3
Challenge Metal borohydrides possess large
weight H2 but are hampered by high decomposition
temperatures (Td). The development of new methods
to
reduce Td (particularly if catalytic) of M(BH4)x
is imperative.1
Using Low valent metal fluorides
Dihydrogen bonding (B-Hd-dH-Y)
Hydrodefluorination (HDF) catalysts are rare and
often suffer from poor activities,1 the rate
limiting factor invariably involves the strong
M-F bond.4
Tds of metal borohydrides (M(BH4)x) are reduced
by addition of a protic species. Loss of H2 now
proceeds by dehydrocoupling (combination of Hd
and Hd-).
Need to destabilise M-F bond. Target electron
rich, low coordinate, high spin metal (I)
fluorides.
Target
Structures of mixed protic/hydridic species are
dominated by inter/intramolecular dihydrogen
bonding,2 that lowers the Td by weakening B-H
bonds and reducing the barrier to reaction.3
Mg(BH4)2 Mg(BH4)2(NH3)2
Td gt 270 ºC Td 150 ºC
MI-F species are of interest not just for HDF,
but for other reactivity due to weakened M-F
bond e.g., alkene insertions.
Rosseinsky and Ingleson developed an unusual
C-Hdd-H-B containing material.4
Using Boron Superelectrophiles
Structure extended in the 3D by dihydrogen
bonding. Here Mg(BH4)2 reacts at an unusually low
temperature, but not by dehydrocoupling (due to
less acidic C-H)
Silylium cations HDF sp3 C-F bonds, but are
inactive towards sp2 C-F bonds.3
Mg(BH4)2(pyrazine)2 2D coordination
polymer
Can B-Hd-dH-C interactions lower Td?
Model complexes will be used to determine
correlation between the strength of C-Hdd-H-B
interaction (IR/XRD/Computationally) and Td
Borinium cations can activate aliphatic and
aromatic C-F bonds due to extreme
electrophilicity and fluorophilicity.
References (1) C. M. Jensen et al., Chem. Rev.,
2007, 107, Pg4111. (2) R. Custelcean, J. E.
Jackson, Chem Rev., 2001, 101, Pg1963. (3a) R. H.
Crabtree et al., JACS, 1999, 121, Pg6337 (3b) I.
Manners et al., Inorg. Chem., 2004, 43, 1090. (4)
M. J. Ingleson, M.J. Rosseinsky et al., Angew.
Chem. Int Ed., 2009, 48, Pg 2012.
References (1) H. Amii and K. Uneyama, Chem Rev.
2009, ASAP. (2) P. J. Dunn et al., 2007, Green
Chem., 9, pg411. (3) O. V. Ozerov et al.,
Science, 2008, 321, Pg 1188. (4) P. L. Holland et
al., JACS, 2005, 127, Pg7857.
Use of (RO2)B-H at (1) will enable an analogous
HDF cycle