An overview of cellular respiration - PowerPoint PPT Presentation
1 / 30
Title:
An overview of cellular respiration
Description:
2,4-dinitrophenol (DNP) Lipid-soluble: makes inner membrane permeable to protons ... Explain why DNP caused weight loss. Experimental Evidence for Chemiosmosis ... – PowerPoint PPT presentation
Number of Views:204
Avg rating:3.0/5.0
Title: An overview of cellular respiration
1
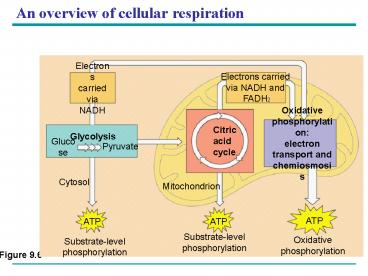
An overview of cellular respiration
2
- Basic Concept The citric acid cycle completes
the energy-yielding oxidation of organic
molecules - The citric acid cycle
- Eight enzyme catalyzed steps
- Occurs in the matrix of the mitochondrion
3
- Before the citric acid cycle can begin
- Pyruvate must first be converted to acetyl CoA,
which links the cycle to glycolysis
4
- An overview of the citric acid cycle
5
Citric Acid Cycle Bottom Line
- The conversion of each acetyl CoA in the citric
acid cycle results in - One ATP produced by substrate-level
phosphorylation - Three NAD reduced to NADH
- One FAD reduced to FADH2
- Two CO2 molecules
6
Figure 9.12
7
The Electron Transport Chain
- Basic Concept Electron transport extracts energy
from high energy electrons to synthesize ATP - NADH and FADH2
- Donate electrons to the electron transport chain
(ETC), which lose energy in several steps
8
Electron TransportChain
9
ETC
- Each successive carrier has a greater
electronegativity than the previous one - At the end of the chain electrons are passed to
oxygen, forming water
10
- Chemiosmosis
- Is an energy-coupling mechanism that uses energy
in the form of a H gradient across a membrane to
drive cellular work
11
Chemiosmosis
- Peter Mitchell (1961) hypothesized that
- At certain steps along the electron transport
chain - Electron transfer causes proteins to pump H from
the mitochondrial matrix to the intermembrane
space - This produces a proton gradient (i.e.,
electrochemical gradient).
12
- The resulting H gradient across the IMM
- Stores energy
- Drives chemiosmosis in ATP synthase
- Is referred to as a proton-motive force
13
(No Transcript)
14
(No Transcript)
15
E. M. of Mitochondrial Inner Membrane Proteins
16
ATP Synthase Molecules
17
ATP Synthase Molecule
18
Chemiosmosis and the electron transport chain
19
Experimental Evidence for Chemiosmosis
- Measured pH of mitochondrial matrix relative to
IMS (space) in metabolically active mitochondria - Predict relative pH of IMS (space) _______
20
Experimental Evidence for Chemiosmosis
- Isolated mitochondria and incubated in vitro with
O2, ADP, PO4 but no e- donors (no NADH or FADH2),
thus, no electron transport. - Exp a Added NADH to incubation.
- Predict what would happen to pH of IM space.
- Exp. b Added excess H (acid) to incubation.
- Predict what would happen to ATP syn.
21
Experimental Evidence for Chemiosmosis
- 2,4-dinitrophenol (DNP)
- Lipid-soluble makes inner membrane permeable to
protons - Binds protons carries them across membrane into
matrix - Abolishes proton gradient across IMM
- In 1940s used as diet pill (until patients died)
- Explain why DNP caused weight loss
22
Experimental Evidence for Chemiosmosis
- In 1997, found mitochondrial protein acting as
natural (endogenous) uncoupling protein (UCP) - Makes IMM permeable to protons
- Uncoupling protein found in inner mitochondrial
membrane of different cells (e.g., adipocytes,
muscle) - Abolishes proton gradient across IMM
- May help determine basal metabolic rate may be
clue to physiological basis of obesity
23
Chemiosmosis The Energy-Coupling Mechanism
- ATP synthase is the enzyme that actually makes ATP
24
(No Transcript)
25
(No Transcript)
26
An Accounting of ATP Production by Cellular
Respiration
- During respiration, most energy flows in the
following sequence - Glucose ? NADH ? electron transport chain ?
proton-motive force ? ATP
27
- There are three main processes in this metabolic
enterprise
28
- Each NADH from the citric acid cycle and
glycolysis contributes enough energy to generate
approximately 3 ATP (rounding up). - Each FADH2 from the citric acid cycle can be used
to generate about 2 ATP. - This plus the 4 ATP from substrate-level
phosphorylation gives a bottom line of 36 - 38
ATP.
29
How efficient is respiration in generating ATP?
- Complete oxidation of glucose releases 686 kcal
per mole. - Formation of each ATP requires at least 7.3
kcal/mole. - Efficiency of respiration is 7.3 kcal/mole x 38
ATP/glucose/686 kcal/mole glucose ? 40 of the
energy in a glucose molecule is captured in ATP - The other approximately 60 is lost as heat.
30
The Evolutionary Significance of Glycolysis
- Glycolysis
- Can produce ATP with or without oxygen, in
aerobic or anaerobic conditions - Occurs in nearly all organisms
- Probably evolved in ancient prokaryotes before
there was oxygen in the atmosphere