ACTIVITY: - PowerPoint PPT Presentation
ACTIVITY:
Activity coefficient for a gas is FUGACITY COEFFICIENT. Below P = 1atm, =1. Deviation from ideal gas behavior. deviations from unity for fugacity ... – PowerPoint PPT presentation
Title: ACTIVITY:
1
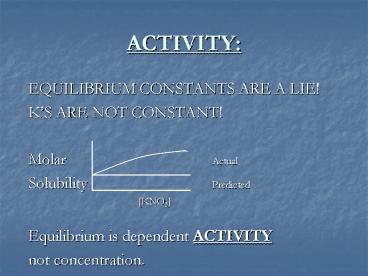
ACTIVITY
- EQUILIBRIUM CONSTANTS ARE A LIE!
- KS ARE NOT CONSTANT!
- Molar Actual
- Solubility Predicted
- KNO3
- Equilibrium is dependent ACTIVITY
- not concentration.
2
AN EXAMPLE
- THE BIG PICTURE Hg2(IO3)2
-
Hg KNO3 - 6.9 x 10-7
M 0 - 1.0 x 10-6
M 0.050 M
3
THE SMALL PICTURE
- PURE
SOLVENT - ANIONS
- CATION
-
IONIC ATMOSPHERE - HIGH TOTAL
-
ION CONCENTRATION - SMALLER IONIC RADIUS
4
ACTIVITIES
- Account for total ion concentration
- aA bB ?? cC dD
- REAL K
- EQUILIBRIUM
- CONSTANT
- Activities
Activity -
Coefficient
5
ACTIVITY COEFFICIENTS FOR CHARGED IONS
Extended Debye Huckel Equation
- Z Charge
- ? Ionic Strength
- ? - Effective size of hydrated ion (pm)
- See Table 6-1 page 91
6
(No Transcript)
7
(No Transcript)
8
Calculations
- Calculate the ionic strength of a 0.023 M
solution of Mg(NO3)2.
9
- Use activities to calculate the molar solubility
of lead iodide, PbI2, in a solution with an ionic
strength of 0.050 M. - Ksp PbI2 7.9 X 10-9
10
ACTIVITY COEFFICIENTS OF NONIONIC COMPOUNDS
- Neutral species have no ionic atmosphere
- Activity Coefficient 1 for dilute solutions.
- lt 1M
11
GASES
- Activity coefficient for a gas is FUGACITY
COEFFICIENT - Below P 1atm, 1
- Deviation from ideal gas behavior ?
- deviations from unity for fugacity
PowerShow.com is a leading presentation sharing website. It has millions of presentations already uploaded and available with 1,000s more being uploaded by its users every day. Whatever your area of interest, here you’ll be able to find and view presentations you’ll love and possibly download. And, best of all, it is completely free and easy to use.
You might even have a presentation you’d like to share with others. If so, just upload it to PowerShow.com. We’ll convert it to an HTML5 slideshow that includes all the media types you’ve already added: audio, video, music, pictures, animations and transition effects. Then you can share it with your target audience as well as PowerShow.com’s millions of monthly visitors. And, again, it’s all free.
About the Developers
PowerShow.com is brought to you by CrystalGraphics, the award-winning developer and market-leading publisher of rich-media enhancement products for presentations. Our product offerings include millions of PowerPoint templates, diagrams, animated 3D characters and more.