Powders Production - PowerPoint PPT Presentation
1 / 31
Title:
Powders Production
Description:
Mechanical Comminution is possible by methods such as impact, attrition, ... processes are based on the conventional processes used in extractive metallurgy. ... – PowerPoint PPT presentation
Number of Views:1346
Avg rating:3.0/5.0
Title: Powders Production
1
Powders Production
2
Powders production
- The significant manufacturing methods may be
classed as follows - Mechanical Methods
- Chemical Methods
- Physical Methods
3
Mechanical Methods
- These processes are not much used as primary
methods for the production of metal powders.
Mechanical Comminution is possible by methods
such as impact, attrition, shear and compression.
The formation of metal powders by mechanical
methods relies on various combinations of these
four basic mechanisms. Such methods have been
used as the primary process for the following
cases - materials which are relatively easy to fracture
such as pure antimony and bismuth, relatively
hard and brittle metal alloys and ceramics. - reactive materials such as beryllium and metal
hydrides. - common metals such as aluminium and iron which
are required sometimes in the form of flake
powder.
4
Mechanical Methods
- Ball Mill
- The ball mill is a key equipment to grind the
crushed materials, and the ball mill is widely
used in powder-making production line including
cement, silicate, new-type building material,
refractory material, fertilizer, ore dressing of
ferrous metal and non-ferrous metal, glass
ceramics, etc, and the ball mill can grind
various ores and other materials with dry type
and wet type.
5
Powders production
- Chemical Methods
- This method can be further classified as
- 1- Chemical reduction
- a) from the solid state
- b) from the gaseous state
- c) from the aqueous solution
- 2-Decomposition
- a) Decomposition of metal hydrides
- b) Decomposition of metal carbonyls
6
Chemical Reduction
- From the solid state
- Chemical reduction involves chemical compound
most frequently an oxide, but sometimes a halide
or other salt of the metal. The reduction
processes are based on the conventional processes
used in extractive metallurgy. Metals are widely
present in nature as sulphides or oxides.
Sulphites can be converted into oxides by tostion
(oxygen is highly reactive with sulphur), while
oxides can be reduced by carbon at high
temperature. Iron powder can be obtained in this
way, but especially if the oxide used has a high
iron content.
7
Chemical Reduction
- The most important reduction plant to produce
sponge iron powder is located at Höganäs (in
Sweden). The raw materials are a mixture of coke,
lime (1) and iron oxide (magnetite, Fe3O4) in the
form of ore (2). After drying (3), crushing (4),
screening (5) and magnetic separation (6) the raw
materials are charged into porous ceramics
retorts (7). The retorts are packed on cars and
run through a tunnel kiln (8) which is several
hundred meters long. The temperature in the kiln
is 1200 ºC and the journey takes about two days.
The product from the reduction process is a
hollow cylinder of coherent raw sponge iron,
which is further processed to molding grade iron
powder. This sponge iron powder is porous
(spongy) and irregular. These particles exhibit
a high strength in powder compaction therefore
the powder is particularly suitable for
compacting into delicate shapes. Reduction takes
place inside the ceramic retort. The iron oxide
is reduced in two steps by carbon monoxide CO
from Fe3O4 to FeO and then to Fe. The CaO is used
as flux in order to reduce the sulphur content
from the sponge iron.
8
Chemical Reduction
9
Chemical Reduction
10
Chemical Reduction
From the gaseous state as in the reduction of
titanium tetrachloride vapor with molten
magnesium the well-known Kroll process.
Refined rutile (or ilmenite) from the ore is
reduced with petroleum-derived coke in a
fluidized bed reactor at 1000 C. The mixture is
then treated with chlorine gas, affording
titanium tetrachloride TiCl4 and other volatile
chlorides, which are subsequently separated by
continuous fractional distillation. In a separate
reactor, the TiCl4 is reduced by liquid magnesium
(15-20 excess) at 800-850 C in a stainless
steel retort to ensure complete
reduction 2Mg(l) TiCl4(g) ? 2MgCl2(l) Ti(s)
T 800-850 C Complications result from
partial reduction of the titanium to its lower
chlorides TiCl2 and TiCl3. The MgCl2 can be
further refined back to magnesium. The resulting
porous metallic titanium sponge is purified by
leaching or heated vacuum distillation. The
sponge is jackhammered out, crushed, and pressed
before it is melted in a consumable electrode
vacuum arc furnace. The melted ingot is allowed
to solidify under vacuum. It is often remelted to
remove inclusions and ensure uniformity. These
melting steps add to the cost of the product.
Titanium is about six times as expensive as
stainless steel.
11
Chemical Reduction
12
Chemical Reduction
http//www.eng.hokudai.ac.jp/labo/ecopro/rosuzuki/
gakkai/00gakkai/PM2KTiMg.html
13
Chemical Reduction
14
Chemical Reduction
15
Chemical Reduction
From the aqueous solution as in the
precipitation of cement copper from copper
sulphate solution with iron or in the reduction
of an ammoniacal nickel salt solution with
hydrogen under pressure (hydrometallurgical
method). Low cost copper powder is produced from
solution obtained by leaching copper ores or
copper scrap, where the precipitation of copper
powder from an acidified solution of copper
sulphate with iron is achieved. Large quantities
of this cement copper are produced from the
copper sulphate solutions which are a by-product
of the copper refinery industry. Most of this
cement copper is eventually melted and cast
rather than used as powder for two reasons (i)
the cement copper produced as a by-product is
rather impure unless special precautions are
taken and (ii) the powder is quite fluffy, i.e.
it has a low apparent density, which is not
satisfactory for many copper powder applications.
To make the powder suitable, a furnace treatment
which would increase the cost would be
necessary.
16
Chemical Decomposition
- Under this category of powder production two
methods are very common. These are - Decomposition of metal hydrides
- Decomposition of metal carbonyls
17
Chemical Decomposition
Decomposition of metal hydrides This involves
first hydriding the refractory metals like Ti,
Zr, Hf, V, Th or U by heating the metal in the
form of sponge, chip or turnings or even compact
metal in hydrogen. TiH2 is formed from titanium
in the temperature range between 300500oC. These
hydrides are quite brittle and can be readily
ball-milled into powder of the desired fineness.
These may be dehydrided by heating them in a good
vacuum at the same temperature at which the
hydride was formed. Care must be taken to avoid
contamination of O2, N2 and C during hydriding or
dehydriding. Uranium hydride may serve as
intermediate not only in producing uranium metal
powder, but also UC and UN powder.
18
Chemical Decomposition
Decomposition of metal carbonyls The famous
example under this category is iron and nickel
powder production. The carbonyls are liquids at
normal temperature with a low boiling point.
These are formed by reaction of the metal and
carbon-monoxide gas under pressure. For example,
iron carbonyl (Fe(CO)5) is formed at 70200
atmosphere pressure and a temperature of
200220oC. The carbonyls can now be decomposed by
heating the vapor at atmospheric pressure. The
usual carbonyl iron powder particles are
spherical with an onion skin structure,
19
Physical Methods
- This method can be further classified as
- 1-Electrolytic Method
- 2-Atomization
- a) Gas atomization
- b) Water atomization
- c) Centrifugal atomization
20
Electrolytic Method
- It is used extensively in the preparation of
copper, beryllium, iron and nickel powders. - The following are the factors promoting powdery
deposits - (a) high current densities
- (b) weak metal concentrations
- (c) additions of colloids and acids
- (d) low temperature
- (e) high viscosities
- (f) avoidance of agitation
- (g) suppression of convection.
21
Electrolytic Method
Schematic of the electrolytic process for making
metal powders
22
Electrolytic Method
23
Atomization
- Arguably the most important method used to
produce metal powders. Atomizing a molten metal
requires much less energy than disintegrating a
solid metal. This process also allows plain
metals and alloys to be produced (so we can
obtain fully prealloyed powders of any alloy that
can be melted). Gas and water atomization are the
main processes used. - 1-Gas Atomization
- 2-Water atomization
24
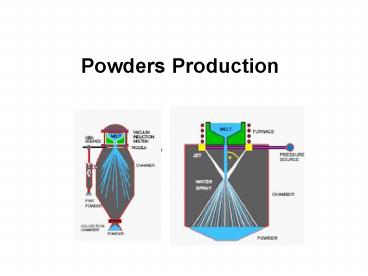
Atomization
- Gas Atomization
- In gas atomization, the medium used to
disintegrate the molten metal could be an inert
gas (like Ar), air, nitrogen or any other
suitable gas. Usually, atomization is performed
in vertical units. - The powders obtained are usually fully spherical
and due to the high pressure of the process, the
size should be lower than 50 µm. The process
usually produces high purity powders. These
powders are not suitable for die pressing, but
are very useful in processes which involve
temperature and pressure at the same time.
25
Atomization
26
Atomization
- Water atomization
- In water atomization, the molten metal leaves the
crucible through an opening and is and then is
disintegrated by high pressure water jets from
the sides. Varying the water pressure, the nozzle
diameter and the angle of the water jets will
control the size and distribution of the powders
obtained. However, these will always be larger
than those obtained by gas atomization. This is
the most popular method of producing iron and
steel powders for many different kinds of PM
products. The contact of the water with the
molten iron produces a high level of oxidation
that forces the reduction annealing of the
powders after drying. Water atomizing units are
usually vertical and the obtained powders have an
irregular shape. These powders have high
compressibility with sufficient green strength
and are widely used for manufacturing structural
sintered components.
27
Atomization
28
Atomization
Two fluid atomization design
29
Atomization
Centrifugal atomization The basis of centrifugal
atomization is the ejection of molten metal from
a rapidly spinning container, plate or disc. The
rotating electrode process (REP) is a further
example of centrifugal atomization.
30
Atomization
Tungsten contamination from the stationary
electrode is a limitation of REP powders. To
eliminate this, the PREP (Plasma Rotating
Electrode Process) method has been
commercialized.
31
Selection of Metal Powder Production Method
- Selection of the production method for a
particular metal powder would depend on - Raw Material Available
- Type of End Application