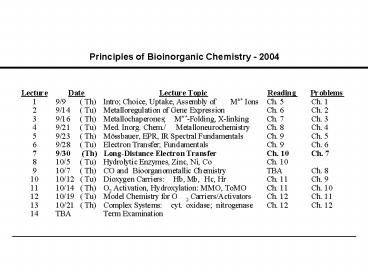
Principles of Bioinorganic Chemistry - 2004 - PowerPoint PPT Presentation
Title:
Principles of Bioinorganic Chemistry - 2004
Description:
Mueller, Biochemistry, 41, 42-51 (2002) Each trace is fit as a sum of exponentials giving rise to the reported rate constants. ... – PowerPoint PPT presentation
Number of Views:48
Avg rating:3.0/5.0
Title: Principles of Bioinorganic Chemistry - 2004
1
Principles of Bioinorganic Chemistry - 2004
2
Long-Distance Electron Transfer in Proteins
Three ways to measure
1. Self-exchange 2. Artificial donor-acceptor
pairs 3. Study of natural protein redox pairs
RedAz
OxAz
OxAz
RedAz
CuI CuII
CuII CuI
for azurin
k 1.3 x 106 M-1 s-1
3
Artificial Donor-Acceptor Pairs
Cytochrome c Fe---Ru, 12 Å
4
Method for Studying ET of Ru-Modified Proteins
Rate 30 s-1, T-independent
5
Distance and Driving Force Dependencies of ET
Rates
6
Distance Dependence from the TDATerm for Reaction
Center
7
Distance dependence from the TDA term for
Ru-modified cytochrome c b from the slope is 1.4
Å-1. Get a 10-fold decrease in rate for every
1.7 Å increase in distance For comparison, b for
ET in vacuum is 2.8 Å-1 and b for ET through
covalent bonds is 0.7 Å-1
(thanks to Brian Crane for the plot)
8
Driving Force Dependence
Data are from ruthenium-modified cytochrome c
derivatives (upper) and a series of covalently
linked donor/acceptor compounds
9
The Mineral Springs in Bath, England, Source of
Methylococcus capsulatus (Bath)
The Restutive Contents of the WATERs Concoctive
Power Solution of gaffes, chaos of Salts and
mineral effluvia of subterranean expiration. It
cleanses the body from all blotches, scurvicial
itchings and BREAKING OUTS WHATSOEVER!
10
(No Transcript)
11
NMR Structure of the Fd Domain of MMOR Mueller,
Biochemistry, 41, 42-51 (2002)
12
(No Transcript)
13
Each trace is fit as a sum of exponentials giving
rise to the reported rate constants.
14
(No Transcript)
15
These rate constants support the analysis with
the full MMOR (Blazyk Lippard, 2000)
16
Electron Transfer from Flavin Domain to Fd Domain
Electron transfer between the two disconnected
flavin and iron/sulfur domains occurs but at a
significantly reduced rate compared to the intact
MMOR protein. (Blazyk Lippard, 2000)
17
For comparison, ET from 3-electron reduced MMOR
to MMOH is characterized by apparent rate
constants of 100 and 16 s-1.
18
Summary - Points to Remember
- Three major metallic units transfer electrons in
bioinorganic chemistry iron-sulfur clusters
blue copper including the dinuclear CuA and
cytochromes (iron porphyrins). - Electrons can transfer over long distances in
10-15 Å hops . The rate depends on driving
force, distance, and orientation of the reacting
partners. Pathways are important (s gt p gt H-bonds
according to theoretical models). - Electron transfer within and between proteins is
optimized to take advantage of the molecular
switching stations. Included are organic units
such as flavins and inorganic units such as
iron-sulfur clusters, both used in the MMOR
protein.