Unit 7 Equations and Solutions - PowerPoint PPT Presentation
1 / 22
Title:
Unit 7 Equations and Solutions
Description:
Double Replacement Rxns will go if a product is made that leaves the water ... D. Balanced Chemical Equations ... x = 2/3 cup milk. 2. Converting from Moles of ... – PowerPoint PPT presentation
Number of Views:84
Avg rating:3.0/5.0
Title: Unit 7 Equations and Solutions
1
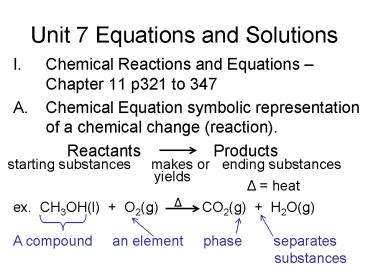
Unit 7 Equations and Solutions
- Chemical Reactions and Equations Chapter 11
p321 to 347 - Chemical Equation symbolic representation of a
chemical change (reaction). - Reactants Products
starting substances makes or ending
substances
yields
? heat
ex. CH3OH(l) O2(g) ? CO2(g)
H2O(g)
A compound an element
phase separates
substances
2
B. Types of Reactions
- Synthesis Rxn makes only one product.
to make
General Form A B ? C
ex. 2Fe(s) O2(g) ? 2FeO(s)
only one kind of product, no sign
2. Decomposition one reactant breaks apart.
General Form A ? B C
ex. 2KClO3(s) ? 2KCl(s) 3O2(g)
note coefficient (number in front of substance)
tells how many of that whole substance you have.
only one kind of reactant
3
- 3. Single Replacement Rxn a more reactive
element replaces a less reactive element in a
compound. Look for a single element on each side
of the reaction.
If C is a nonmetal.
General Form AB C ? AC B
If C is a metal.
or AB C ? CB A
ex. 2NaI Cl2 ? 2NaCl I2
Cl replaces I because Cl is more reactive than I.
ex. LiF K ? KF Li
4
- Double Replacement Rxn A reaction in which
ionic compounds in water (aq) switch partners
to make a product that leaves the water (an
insoluble solid or gas). - General Form AB CD ? AD CB
ex. K2S(aq) ZnCl2(aq) ? 2 KCl(aq) ZnS(s)
5. Combustion Rxn The reaction of a carbon
compound with Oxygen to form Carbon Dioxide and
Water.
(show video glencoe chap6 3)
General Form CxHyOz O2(g) ? CO2(g) H2O
ex. 2CH3OH(g) 3O2(g) ? 2CO2(g) 4H2O(g)
5
C. How To Tell if a Reaction Will Go
- A Rxn is said to go if, when reactants are
combined and activation energy is supplied,
products are made a Spontaneous Rxn. - Single Replacement Rxn A more reactive element
replaces a less reactive element. Table J lists
elements in order from most to least reactive. - In general AB C ? CB A goes if element C
is above element A in Table J. - ex. Will the rxn CuSO4 Zn ? ZnSO4 Cu go?
6
(No Transcript)
7
2. Double Replacement Rxn
- Double Replacement Rxns will go if a product is
made that leaves the water solution either an
insoluble salt (a solid precipitate) or a gas. - Table F lists Guidelines for telling if a salt is
soluble (dissolves) in water. - If one or both products is insoluble the reaction
will go and the insoluble product will form a
precipitate. - ex. Pb(NO3)2(aq) KI(aq) ?
- Will this rxn go and if so what is the ppt.?
8
(No Transcript)
9
D. Balanced Chemical Equations
- What must be equal between the reactants and
products in a chemical equation? - Mass of reactants Mass of products
- The number of atoms of each element on reactant
and product sides must be equal. - This is because of the Law of Conservation of
Mass (Matter). - A balanced equation has equal number of atoms of
each element on each side. - ex. H2(g) Cl2(g) ? 2HCl(g)
10
2. Balancing Equations
- Write out the equation.
- List all elements and the amount on each side
under the equation. - Decide what to multiply by to make amounts of
each element on each side equal write these
multiples in as coefficients. - ex. Li(s) CaCl2(aq) ? LiCl(aq) Ca(s)
- Li 1 1
- Ca 1
1 - Cl 2 1
11
E. Stiochiometry Problems based on Equations
- Converting From Amount of One Substance to Amount
of Another Substance. - Recipe for French Toast 3 eggs
- 1
cup milk etc. - We can adjust the recipe for amounts we have
using a ratio - ex. If we only have 2 eggs, how much milk
do we use?
2 eggs
3 eggs
1 cup milk
x cup milk
3x 2
x 2/3 cup milk
12
- 2. Converting from Moles of One Substance to
Moles of Another Substance. - Chemical Equations are like recipes, coefficients
are the amounts! - ex. Given the balanced equation
- H2(g) Cl2(g) ? 2HCl(g)
- How many molecules of HCl(g) can be made from one
molecule of H2(g) assuming excess Cl2? - How many moles of HCl(g) molecules can be made
from one mole of H2(g) molecules assuming excess
Cl2?
13
Ex. How many moles of HCl can be made from 5.0
moles of H2(g), assuming excess Cl2(g)?
H2 Cl2 ? 2 HCl
Ex. Given the balanced equation
N2(g)3H2(g)?2NH3(g), how many moles of ammonia
can be made from 5.0 moles of H2?
14
II. SOLUTIONS Chapter 16, pgs 320-333
- Terms to know
- Solution a homogenous mixture.
- Solvent substance in which other substances
dissolve the substance in greater amount. - Solute a substance that is dissolved the
substance in lesser amount. - Concentration amount of solute dissolved per
unit of solvent. Dilute small
amount Concentrated
relatively large amount
15
B. Saturation and Solubility
- A saturated solution has the maximum amount of
solute dissolved in it. - Unsaturated less than maximum.
- Supersaturated more than maximum?!
- Solubility concentration of a saturated soln.
- Soluble ? Slightly Soluble ? Insoluble
- Solubility changes with temp. and press.(gas)
- Solid solute inc. Temp. inc. Solubility
- Gaseous solute inc. Temp. dec. Solubility
- inc. Press.
inc. Solubility
16
(No Transcript)
17
C. Example Regents Problems Based on Table G
- According to Table G, which solution is saturated
at 30oC? - (1) 6 g of KClO3 in 100g of H2O
- (2) 24g of KClO3 in 200g of H2O
- (3) 15g of NaCl in 100g of H2O
- (4) 30g of NaCl in 200g of H2O
- 100g of H2O is saturated with NH4Cl at 50oC.
According to Table G, if the temperature is
lowered to 10oC, what is the total amount of
NH4Cl that will precipitate? - (1) 5.0g (2) 30.g (3) 17g
(4) 50.g
18
D. Molarity Calculations
- Molarity, a concentration unit the number of
moles of solute dissolved per liter of solution.
Moles of solute
n
- Morality
or
M
Liters of solution
L
Ex. What is the molarity of a solution that
contains 4 moles of solute dissolved in 8,000ml
of solution?
4 moles
M x
x
8.0 L
n 4 moles
x 0.5M
L 8,000ml
8.0 L
19
- Ex. What is the molarity of a 4.0 L solution made
from mixing 29g of sodium chloride in water?
moles of solute
M
x
Molarity
Liters of solution
L
4.0 L
0.50moles
n
29g
/(58.5g/mole)
x
4.0 L
0.50mol
x 0.13 M
Ex. What mass of NaOH is needed to make 0.5L of a
6.0M NaOH(aq) solution.
n
M
L
M 6.0M
x
L 0.5 L
6.0M
0.5L
x
3.0
n x
40
x 3.0 moles
m
n
x 120 g
gfm
20
E. Other Methods of Indicating Concentration
volume of solute
- by Volume x 100
- ex. What is the percent by volume concentration
- of 10.ml of ethanol dissolved in 50.ml of water?
- volume of solute 10.ml
- volume of solution
volume of solution
50ml
10.ml 60.ml
10.ml
vol x 100
60.ml
vol 17
21
mass of solute
- by Mass x 100
- ex. What is the percent by mass concentration
- of a solution where 5.0g of salt are dissolved
in 80.g of water? - mass of solute 5.0g
- mass of solution 85g
mass of solution
5.0g
mass x 100
85g
mass 5.9
22
3. Parts per Million ppm
x 1,000,000
mass of solute
mass of solution
ex. Find the concentration in ppm if 0.100 g of
sucrose are dissolved in 500. g of water.
mass of solute 0.100 g
mass of solution 500.1 g
0.100 g
ppm x 1,000,000
500.1 g
ppm 200. ppm
















![Solve Simultaneous Equations One Linear, one quadratic [Circle] PowerPoint PPT Presentation](https://s3.amazonaws.com/images.powershow.com/5861858.th0.jpg?_=20200807031)













