Activity - PowerPoint PPT Presentation
1 / 57
Title:
Activity
Description:
Opposite of common ion effect ... Effect of Ionic Strength on Solubility ... Write a mass balance for a solution of Fe2(SO4)3, if the species are Fe3 , Fe(OH) ... – PowerPoint PPT presentation
Number of Views:140
Avg rating:3.0/5.0
Title: Activity
1
Activity
- Introduction
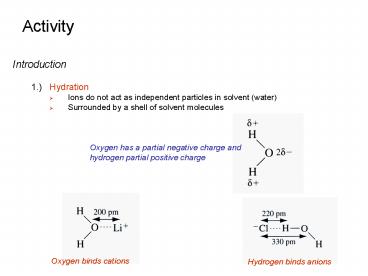
- 1.) Hydration
- Ions do not act as independent particles in
solvent (water) - Surrounded by a shell of solvent molecules
Oxygen has a partial negative charge and hydrogen
partial positive charge
Oxygen binds cations
Hydrogen binds anions
2
Activity
Introduction 2.) H2O exchanges rapidly between
bulk solvent and ion-coordination sites
3
Activity
- Introduction
- 3.) Size of Hydration
- Size and charge of ion determines number of bound
waters - Smaller, more highly charged ions bind more water
molecules - Activity is related to the size of the
hydrated species
Small Ions bind more water and behave as larger
species in solution
4
Activity
- Effect of Ionic Strength on Solubility
- 1.) Ionic Atmosphere
- Similar in concept to hydration sphere
- Cation surrounded by anions and anions are
surrounded by cations - - Effective charge is decreased
- - Shields the ions and decreases attraction
- Net charge of ionic atmosphere is less than ion
- - ions constantly moving in/out of ionic
atmosphere
Each ion-plus-atmosphere contains less net charge
and there is less attraction between any
particular cation and anion
Each ion see less of the other ions charge and
decreases the attraction
5
Activity
- Effect of Ionic Strength on Solubility
- 2.) Ionic Strength (m)
- Addition of salt to solution increases ionic
strength - - Added salt is inert ? does not interact or
react with other ions - In general, increasing ionic strength increases
salt solubility - - Opposite of common ion effect
The greater the ionic strength of a solution, the
higher the charge in the ionic atmospheres
More ions added, more ions can be present in
ionic atmospheres
6
Activity
- Effect of Ionic Strength on Solubility
- 2.) Ionic Strength (m)
- Measure of the total concentration of ions in
solution - - More highly charged an ion is the more it is
counted - - Sum extends over all ions in solution
where Ci is the concentration of the ith species
and zi is its charge
7
Activity
- Effect of Ionic Strength on Solubility
- 2.) Ionic Strength (m)
- Example What is the ionic strength of a 0.0087
M KOH and 0.0002 M La(IO3)3 solution? Assume
complete dissociation and no formation of LaOH2
8
Activity
- Effect of Ionic Strength on Solubility
- 3.) Equilibria Involving Ionic Compounds are
Affected by the Presence of All Ionic Compounds
in the Solution - Knowing the ionic strength is important in
determining solubility - Example
- Ksp 1.3x10-18
If Hg2(IO3)2 is placed in pure water, up to
6.9x10-7M will dissolve. If 0.050 M KNO3 is
added, up to 1.0x10-6M Hg2(IO3)2 will dissolve.
Occurs Due to Changes in the Ionic Strength
Activity Coefficients
9
Activity
- Equilibrium Constant and Activity
- 1.) Typical Form of Equilibrium Constant
- However, this is not strictly correct
- Ratio of concentrations is not constant under all
conditions - Does not account for ionic strength differences
- 2.) Activities, instead of concentrations should
be used - Yields an equation for K that is truly constant
where AA, AB, AC, AD is activities of A through D
10
Activity
- Equilibrium Constant and Activity
- 3.) Activities account for ionic strength effects
- Concentrations are related to activities by an
activity coefficient (g) - 4.) Real Equilibrium Constant Using Activity
Coefficients
where AC is activity of C C is
concentration of C gC is activity coefficient of
C
11
Activity
- Equilibrium Constant and Activity
- 4.) Real Equilibrium Constant Using Activity
Coefficients - g is always 1
- Activity coefficient measures the deviation from
ideal behavior - - If g 1, the behavior is ideal and typical form
of equilibrium constant is used - Activity coefficient depends on ionic strength
- - Activity coefficient decrease with increasing
ionic strength - - Approaches one at low ionic strength
Activity depends on hydrated radius (a) of the
ion. This includes the ion itself and any water
closely associated with it.
12
Activity
- Equilibrium Constant and Activity
- 5.) Activity Coefficients of Ions
- Extended Debye-H?ckel Equation
- Only valid for concentrations 0.1M
- In theory, a is the diameter of hydrated ion
where g is the activity coefficient a is
ion size (pm) z is the ion charge m is the
ionic strength
13
Activity
- Equilibrium Constant and Activity
- 5.) Activity Coefficients of Ions
- In practice, a is an empirical value, provide
agreement between activity and ionic strength - - sizes can not be taken literally
- - trends are sensible ? small, highly charged
ions have larger effective sizes - a Li gt Na gt K gt Rb
Ideal behavior when g 1 - low ionic
strength - low concentration - low charge/large
a
14
Activity
Activity Coefficients from Debye-H?ckel Equation
15
Activity
- Equilibrium Constant and Activity
- 6.) Example 1
- What is the activity coefficient of Hg22 in a
solution of 0.033 M Hg2(NO3)2?
Solution Step 1 Determine m
16
Activity
- Equilibrium Constant and Activity
- 6.) Example 1
- What is the activity coefficient of Hg22 in a
solution of 0.033 M Hg2(NO3)2?
Solution Step 2 Identify Activity Coefficient
from table at corresponding ionic strength.
17
Activity
- Equilibrium Constant and Activity
- 6.) Example 2
- What is the activity coefficient for H at m
0.025 M?
Note Values for g at m 0.025 are not listed in
the table.
There are two possible ways to obtain g in this
case a.) Direct Calculation (Debye-H?ckel)
zH
m
a for H from table
18
Activity
- Equilibrium Constant and Activity
- 6.) Example 2
- What is the activity coefficient for H at m
0.025 M? - b.) Interpolation
- Use values for gH given at m 0.01 and 0.05 m
from table and assume - linear change in g with m.
To solve for gH at m 0.025
Fract. Of Interval Between 0.01 and 0.05
Diff. in g values at 0.01 and 0.05
gH at m 0.01
19
Activity
- Equilibrium Constant and Activity
- 6.) Example 2
- What is the activity coefficient for H at m
0.025 M? - b.) Interpolation
- Use values for gH given at m 0.01 and 0.05 m
from table and assume - linear change in g with m.
Note This value is slightly different from the
calculated value (0.88) since it is only an
estimate.
20
Activity
- Equilibrium Constant and Activity
- 7.) Activity Coefficients of Gasses and Neutral
Molecules - For nonionic, neutral molecules
- - g 1 for m 0.1 M
- - or Ac C
- For gases,
- - g 1 for pressures 1 atm
- - or A P, where P is pressure in atm
- 8.) Limitation of Debye-H?ckel Equation
- Debye-H?ckel predicts g decreases as m increases
- - true up to m 0.10 M
- At higher m, the equation is no longer accurate
- - at m 0.5 M, most ions actually show an
increase in g - with an increase in m
- - at higher m, solvent is actually a mixture
instead of just - water
Hydration sphere is mixture of water and salt at
high concentration
21
Activity
- pH
- 1.) When we measure pH with a pH meter, we are
measuring the negative logarithm of the hydrogen
ion activity - Not measuring concentration
- 2.) Affect of pH with the Addition of a Salt
- Changes ionic strength ? Changes H and OH-
activity
22
Activity
- pH
- 2.) Affect of pH with the Addition of a Salt
- Example
- What is the pH of a solution containing 0.010M
HCl plus 0.040 M KClO4?
23
Activity
- Using Activity Coefficients
- 1.) Activity Coefficients Need to be Considered
for Accurate Answers Involving Equilibrium
Constants - Example 1
What is the Hg22 in a saturated solution of
Hg2Br2 with 0.00100M KCl, where and KCl acts
as an inert salt?
Ksp 5.6x10-23
24
Activity
- Using Activity Coefficients
- 1.) Activity Coefficients Need to be Considered
for Accurate Answers Involving Equilibrium
Constants - Example 2
What is the Hg22 in a saturated solution of
Hg2Br2 with 0.00100M KBr?
Note KBr is not an inert salt, since Br- is also
present in the Ksp reaction of Hg2Br2
25
Activity
- Using Activity Coefficients
- 1.) Activity Coefficients Need to be Considered
for Accurate Answers Involving Equilibrium
Constants - Example 3
What is the true concentration of Li and F- in a
saturated solution of LiF in water?
Note Only LiF is present in solution. Ionic
strength is only determined by the amount of LiF
that dissolves
Solution Set-up the equilibrium equation in
terms of activities
26
Activity
- Using Activity Coefficients
- 1.) Activity Coefficients Need to be Considered
for Accurate Answers Involving Equilibrium
Constants - Example 3
Note Both x and gLi,gF- depend on the final
amount of LiF dissolved in solution
To solve, use the method of successive of
approximation
Solution Assume gLi gF- 1. Solve for x.
27
Activity
- Using Activity Coefficients
- 1.) Activity Coefficients Need to be Considered
for Accurate Answers Involving Equilibrium
Constants - Example 3
Solution Step 2 use the First Calculated Value
of Li and F- to Estimate the Ionic
Strength and g Values.
Obtained by using m0.041 and interpolating data
in table
28
Activity
- Using Activity Coefficients
- 1.) Activity Coefficients Need to be Considered
for Accurate Answers Involving Equilibrium
Constants - Example 3
Solution Step 3 use the calculated values for
gF and gLi to re-estimate Li and F-.
substitute
29
Activity
- Using Activity Coefficients
- 1.) Activity Coefficients Need to be Considered
for Accurate Answers Involving Equilibrium
Constants - Example 3
Solution Repeat Steps 2-3 Until a Constant
Value for x is obtained
For this example, this occurs after 3-4 cycles,
where x 0.050M
gF, gLi
Use g to calculate new concentrations.
Use concentrations to calculate new m and g.
F-, Li
30
Equilibrium
- Systematic Treatment of Equilibrium
- 1.) Help Deal with Complex Chemical Equilibria
- Set-up general equations
- Simplify using approximations
- Introduce specific conditions ? number of
equations number of unknowns - 2.) Charge Balance
- The sum of the positive charges in solution
equals the sum of the negative charges in
solution.
(positive charge)
(negative charge)
where C is the concentration of a cation n
is the charge of the cation A is the
concentration of an anion m is the charge of
the anion
A solution will not have a net charge!
31
Equilibrium
- Systematic Treatment of Equilibrium
- 2.) Charge Balance
- If a solution contains the following ionic
species H, OH-,K,H2PO4-,HPO42- and PO43-, the
charge balance would be
The coefficient in front of each species
always equals the magnitude of the charge
on the ion.
For a solution composed of 0.0250 mol of KH2PO4
and 0.0300 mol of KOH in 1.00L
H 5.1x10-12M H2PO4- 1.3x10-6 M K
0.0550 M HPO42- 0.0220M OH-
0.0020M PO43- 0.0030M
Charge balance
32
Equilibrium
- Systematic Treatment of Equilibrium
- 3.) Mass Balance
- Also called material balance
- Statement of the conservation of matter
- The quantity of all species in a solution
containing a particular atom must equal the
amount of that atom delivered to the solution
Acetic acid
Acetate
Mass balance for 0.050 M in water
Include ALL products in mass balance H3PO4?
H2PO4-,HPO42-, PO43-
33
Equilibrium
- Systematic Treatment of Equilibrium
- 3.) Mass Balance
- Example 1
Write the mass balance for a saturated solution
of the slightly soluble salt Ag3PO4, which
produces PO43- and Ag when it dissolves.
Solution If phosphate remained as PO43-,
then but, PO43- reacts with water
34
Equilibrium
- Systematic Treatment of Equilibrium
- 3.) Mass Balance
- Example 2
Write a mass balance for a solution of Fe2(SO4)3,
if the species are Fe3, Fe(OH)2, Fe(OH)2,
Fe2(OH)24, FeSO4, SO42- and HSO4-.
35
Equilibrium
- Systematic Treatment of Equilibrium
- 1.) Write all pertinent reactions.
- 2.) Write the charge balance equation.
- Sum of positive charges equals the sum of
negative charges in solution - 3.) Write the mass balance equations. There may
be more than one. - Conservation of matter
- Quantity of all species in a solution containing
a particular atom must equal the amount of atom
delivered to the solution - 4.) Write the equilibrium constant expression for
each chemical reaction. - Only step where activity coefficients appear
- 5.) Count the equations and unknowns
- Number of unknowns must equal the number of
equations - 6.) Solve for all unknowns
36
Equilibrium
- Applying the Systematic Treatment of Equilibrium
- 1.) Example 1
- Ionization of water
Kw
Kw 1.0x10-14 at 25oC
Step 1 Pertinent reactions
Step 2 Charge Balance
Step 3 Mass Balance
H2O, H, OH- determined by Kw
Not True!
37
Equilibrium
- Applying the Systematic Treatment of Equilibrium
- 1.) Example 1
- Ionization of water
Step 4 Equilibrium constant expression
Step 5 Count equations and unknowns
Two equations
(1)
(2)
Two unknowns
(1)
(2)
38
Equilibrium
- Applying the Systematic Treatment of Equilibrium
- 1.) Example 1
- Ionization of water
Step 6 Solve
Ionic strength (m) of pure water is very low, gH
and gOH- 1
substitute
39
Equilibrium
- Applying the Systematic Treatment of Equilibrium
- 2.) Example 2
- Solubility of Calcium Sulfate
- Find concentrations of the major species in a
saturated solution of CaSO4
Step 1 Pertinent reactions
This information is generally given
40
Equilibrium
- Applying the Systematic Treatment of Equilibrium
- 2.) Example 2
- Solubility of Calcium Sulfate
- Find concentrations of the major species in a
saturated solution of CaSO4
Step 2 Charge Balance
Step 3 Mass Balance
Doesnt matter what else happens to these ions!
41
Equilibrium
- Applying the Systematic Treatment of Equilibrium
- 2.) Example 2
- Solubility of Calcium Sulfate
- Find concentrations of the major species in a
saturated solution of CaSO4
Step 4 Equilibrium constant expression (one
for each reaction)
42
Equilibrium
- Applying the Systematic Treatment of Equilibrium
- 2.) Example 2
- Solubility of Calcium Sulfate
- Find concentrations of the major species in a
saturated solution of CaSO4
Step 5 Count equations and unknowns
Seven Equations
(1)
(CB)
(2)
(MB)
(3)
(4)
(6)
(5)
(7)
Seven Unknowns
43
Equilibrium
- Applying the Systematic Treatment of Equilibrium
- 2.) Example 2
- Solubility of Calcium Sulfate
- Find concentrations of the major species in a
saturated solution of CaSO4
Step 6 Solve (Not Easy!) - dont know ionic
strength ? dont know activity coefficients -
where to start with seven unknowns
- Make Some Initial Assumptions
- At first, set all activities to one to calculate
ionic strength
- HOH-1x10-7, remaining chemical reactions
are independent of water
- At first, ignore equations with small
equilibrium constants
44
Equilibrium
- Applying the Systematic Treatment of Equilibrium
- 2.) Example 2
- Solubility of Calcium Sulfate
- Find concentrations of the major species in a
saturated solution of CaSO4
Step 6 Solve (Not Easy!)
- Assumptions Reduce Number of Equations and
Unknowns - Three unknowns
- Three equations
Mass balance and charge balance reduces to
Charge balance
Mass balance
H OH-
Low concentrations ? small equilibrium constant
Low concentrations ? small equilibrium constant
Simple Cancellation
45
Equilibrium
- Applying the Systematic Treatment of Equilibrium
- 2.) Example 2
- Solubility of Calcium Sulfate
- Find concentrations of the major species in a
saturated solution of CaSO4
Step 6 Solve (Not Easy!)
So, CaSO4 is known
Therefore, only two equations and two unknowns
substitute
and
46
Equilibrium
- Applying the Systematic Treatment of Equilibrium
- 2.) Example 2
- Solubility of Calcium Sulfate
- Find concentrations of the major species in a
saturated solution of CaSO4
Step 6 Solve (Not Easy!)
Given
Determine Ionic Strength
Determine Activity Coefficients
From table
47
Equilibrium
- Applying the Systematic Treatment of Equilibrium
- 2.) Example 2
- Solubility of Calcium Sulfate
- Find concentrations of the major species in a
saturated solution of CaSO4
Step 6 Solve (Not Easy!)
Use activity coefficients and Ksp equation to
calculate new concentrations
Use new concentrations to calculate new ionic
strength and activity coefficients
48
Equilibrium
- Applying the Systematic Treatment of Equilibrium
- 2.) Example 2
- Solubility of Calcium Sulfate
- Find concentrations of the major species in a
saturated solution of CaSO4
Step 6 Solve (Not Easy!)
Repeat process until calculated numbers converge
to a constant value
Stop, concentrations converge
49
Equilibrium
- Applying the Systematic Treatment of Equilibrium
- 2.) Example 2
- Solubility of Calcium Sulfate
- Find concentrations of the major species in a
saturated solution of CaSO4
Step 7 Check Assumptions
With
Both HSO4- and CaOH are 5 times less than
Ca2 and SO42- ? assumption is reasonable
50
Equilibrium
- Applying the Systematic Treatment of Equilibrium
- 2.) Example 3
- Solubility of Magnesium Hydroxide
- Find concentrations of the major species in a
saturated solution of Mg(OH)2
Step 1 Pertinent reactions
Ksp
Ksp 7.1x10-12
K1
K1 3.8x102
Kw
Kw 1.0x10-14
Step 2 Charge Balance
51
Equilibrium
- Applying the Systematic Treatment of Equilibrium
- 2.) Example 3
- Solubility of Magnesium Hydroxide
- Find concentrations of the major species in a
saturated solution of Mg(OH)2
Step 3 Mass Balance (tricky)
OH- 2Mg2
But, two sources of OH-, OH- H
Account for both sources of OH-
Species containing OH-
Species containing Mg
52
Equilibrium
- Applying the Systematic Treatment of Equilibrium
- 2.) Example 3
- Solubility of Magnesium Hydroxide
- Find concentrations of the major species in a
saturated solution of Mg(OH)2
Step 4 Equilibrium constant expression (one
for each reaction)
Proper to write equilibrium equations using
activities, but complexity of manipulating
activity coefficients is a nuisance.
Most of the time we will omit activity
coefficients
53
Equilibrium
- Applying the Systematic Treatment of Equilibrium
- 2.) Example 3
- Solubility of Magnesium Hydroxide
- Find concentrations of the major species in a
saturated solution of Mg(OH)2
Step 5 Count equations and unknowns
Four equations
(1)
CBMB
(2)
(3)
(4)
Four unknowns
54
Equilibrium
- Applying the Systematic Treatment of Equilibrium
- 2.) Example 3
- Solubility of Magnesium Hydroxide
- Find concentrations of the major species in a
saturated solution of Mg(OH)2
Step 6 Solve (Not Easy!)
- Assumption to Reduce Number of Equations and
Unknowns - Solution is very basic OH- gtgt H, neglect
H
CBMB
Rearrange K1 (ignore activity coefficients)
55
Equilibrium
- Applying the Systematic Treatment of Equilibrium
- 2.) Example 3
- Solubility of Magnesium Hydroxide
- Find concentrations of the major species in a
saturated solution of Mg(OH)2
Step 6 Solve (Not Easy!)
Substitute K1 into Mass or Charge Balance
Solve for Mg2
56
Equilibrium
- Applying the Systematic Treatment of Equilibrium
- 2.) Example 3
- Solubility of Magnesium Hydroxide
- Find concentrations of the major species in a
saturated solution of Mg(OH)2
Step 6 Solve (Not Easy!)
Substitute Mg2 into Ksp equation
Reduces to a single equation with a single
variable
Solve using spreadsheet, vary OH- until obtain
correct value for Ksp (7.1x10-12)
57
Excel Demo of Goal Seek