Some thermodynamic parameters of radicals - PowerPoint PPT Presentation
1 / 9
Title:
Some thermodynamic parameters of radicals
Description:
radical reaction step more than compensates for the strain that ... reactivity of starting alkyl halide: RBr or RI (relatively reactive) not RCl (too unreactive) ... – PowerPoint PPT presentation
Number of Views:61
Avg rating:3.0/5.0
Title: Some thermodynamic parameters of radicals
1
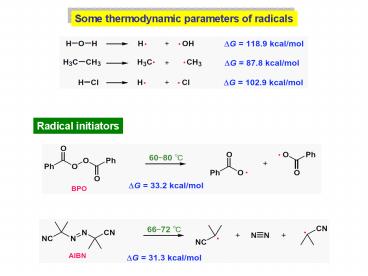
Some thermodynamic parameters of radicals
Radical initiators
2
some persistent (unreactive) radicals
3
Vitamine E tames radicals
Ketyl Radicals
4
ketyl radical anion in aprotic solvents
pinacol reaction
5
McMurry reaction
observed only if the reaction is carried out at
low temperature
6
Acyloin reaction
7
the energy to be gained by pairing up the two
electrons in the radical reaction step more than
compensates for the strain that may be generated
in forming the ring
Carbon-carbon bond formation with radicals
8
initiation
propagation
9
What has something to do with each radical's
selectivity?
bond strength Sn-C bond is relatively weak
the rate constant of Rwith Bu3SnH is about the
same as that for reaction with CH2CHCN should
be CH2CHCN gt 10 x Bu3SnH , which, however,
causes problems with side reactions.
Therefore,Bu3SnH should be added very
slowly. reactivity of starting alkyl
halide RBr or RI (relatively reactive) not RCl
(too unreactive)
hn