Reduction and Oxidation Reactions - PowerPoint PPT Presentation
1 / 32
Title:
Reduction and Oxidation Reactions
Description:
Aromatic halides more difficult to dehalogenate than aliphatic or vinyl ... Dissociative for aliphatic compounds (halogenated methanes and ethanes) CCl4 e ... – PowerPoint PPT presentation
Number of Views:150
Avg rating:3.0/5.0
Title: Reduction and Oxidation Reactions
1
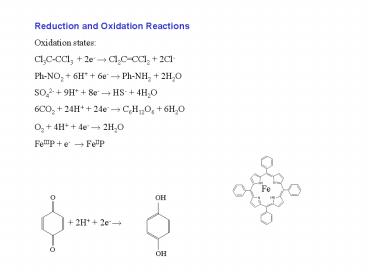
Reduction and Oxidation Reactions Oxidation
states Cl3C-CCl3 2e- ? Cl2CCCl2
2Cl- Ph-NO2 6H 6e- ? Ph-NH2 2H2O SO42-
9H 8e- ? HS- 4H2O 6CO2 24H 24e- ?
C6H12O6 6H2O O2 4H 4e- ? 2H2O FeIIIP e-
? FeIIP
Fe
2
Redox Reactions
- Chapter 14
3
Redox reactions may be abiotic or biological
abiotic means it does not involve a living
organism(but may involve biological molecules
released from cells)
4
Common environmental redox reactions
5
Nernst equation remember DG
-nFE Half-Reactions coupling of oxidation and
reduction reductant FeIIP ? FeIIIP e- E
-0.07 (pH 7) oxidant CCl4 e- ? CCl3 Cl- E
0.09 (favorable) CCl3H e- ? CCl2H Cl- E
-0.19 (not favorable) Note Cl- 10-3 M
6
(No Transcript)
7
Factors affecting redox condition in the
environment Note pE is a rotten way to predict
reaction rates Reduction reactions are a function
of the identity and conc. of reductants present.
8
Reduction potentials
Reductive Dehalogenation More halogens more
favorable redox potential Br easier to reduce
than Cl Aromatic halides more difficult to
dehalogenate than aliphatic or vinyl
9
Oxidation reactions
Most contaminants are stable with respect to
abiotic oxidation (O2 is a kinetically weak
oxidant). Groups that can be abiotically
oxidized include mercaptans R-SH anilines
Ph-NH2 phenols Ph-OH Environmentally
important oxidizers include Fe(III) and
Mn(III/IV) minerals
10
Oxidation of phenols by Mn(III/IV) oxides
form inner-sphere complex surface ? Mn(III)-OH
ArOH ? surface ? Mn(III)-O-Ar H2O electron
transfer surface ? Mn(III)-O-Ar ? surface ?
Mn(II),O-Ar release of phenoxy
radical surface ? Mn(II),O-Ar H2O ? surface
? Mn(II)-OH2 O-Ar release of Mn(II) surface
? Mn(II)-OH2 ? Mn2 (aq) new site Note the
incredibly complicated kinetics, which depend on
pH (speciation of oxide surface sites and
phenol) number of available sites rate at which
they are regenerated affinity of phenol for
sites mass transfer? Stone 1987
11
Reduction reactions Groups that can be reduced
include halogens (I, Br, Cl, usually not
F) nitro (-NO2) Environmentally important
reductants include reduced sulfur species such
as HS- many minerals, esp. Fe(II) minerals (FeS,
FeCO3) Fe(II) adsorbed to mineral surfaces
Electron transfer mediators dissolved,
complexed Fe(II) species quinones
12
(No Transcript)
13
(No Transcript)
14
(No Transcript)
15
(No Transcript)
16
(No Transcript)
17
Reductive dehalogenation Important for nearly
all chlorinated and brominated species. Dissociati
ve for aliphatic compounds (halogenated methanes
and ethanes) CCl4 e- ? CCl3 Cl-
non-dissociative for aromatics Probably
dissociative for vinyl halides Cl2CCCl2 e- ?
Cl2CCCl Cl-
-
e-
Cl-
18
One-electron reduction potentialsmore halogens
more favorable reduction potential
Totten and Roberts, 2001
19
Reduction pathways
Hydrogenolysis
RCl 2e- H
RH Cl -
Reductive b-elimination (usually favored)
H2ClC-CClH2 2e-
H2CCH2 2Cl -
HClCCClH 2e-
HC CH 2Cl -
Reductive a-elimination CCl4 2e- CCl3 - Cl
- CCl2 2Cl -
20
Hypothesized reaction sequence for reduction of
chlorinated ethylenes and related compounds by
Zn(0). (also true for reactions with vitamin B12,
Fe(0), etc.) from Arnold and Roberts, 1998
21
Reductive elimination is thermodynamically
favored over hydrogenolysis from Totten and
Roberts, 2001
22
For reactions of chlorinated ethylenes with
Zn(0), thermodynamic considerations can predict
amount of elimination product formed. (from
Arnold and Roberts 1998)
23
LFERs for reduction reactions E1 (when
available) LUMO BDE (dissociative
reactions) E2s?
24
Correlation of surface area normalized rate
constants (kSA) for reactions of chlorinated
ethylenes with Zn(0) with one-electron reduction
potentials. (from Arnold and Roberts 1998).
25
Correlation of surface area normalized rate
constants (kSA) for reactions of chlorinated
ethylenes with Zn(0) with two-electron reduction
potentials for hydrogenolysis and reductive
elimination. from Arnold and Roberts 1998
26
Zhijie Liu, Eric A. Betterton, and Robert G.
Arnold Electrolytic Reduction of Low Molecular
Weight Chlorinated Aliphatic Compounds
Structural and Thermodynamic Effects on Process
Kinetics, Environmental Science Technology
2000 34(5) 804-811. porous nickel cathode. The
chlorinated ethenes reacted much faster than
predicted from bond enthalpy calculations and the
alkane-based correlation, suggesting that alkenes
are not transformed via the same mechanism as the
chlorinated alkanes. Dihalo-elimination was the
predominant pathway for reduction of vicinal
polychlorinated alkanes. For chlorinated alkenes
and geminal chlorinated alkanes, sequential
hydrogenolysis was the major reaction pathway.
alkenes
alkanes
27
Correlation of the kinetic parameter for
chlorinated ethylene reduction by Fe(0) with
one-electron reduction potential (E1). Whats
wrong with this picture?! From Arnold and
Roberts, 2000
28
Reductive dechlorination of PCBs
(aromatics) occurs in anaerobic biodegradation,
but slow reduces toxicity but doesnt destroy
backbone more chlorines more favorable
reduction potential, but very little
thermodynamic data available Regiospecificity bac
teria generally dehalogenate meta and para
positions Woods, Trobaugh, Carter, EST 1999--a
model reductant, (vitamin B12s ) dehalogenated
all positions equally (products were predicted
based on thermodynamic calculations)
29
Microbial dechlorination of PCBs
- So far, seen only in aquatic sediments
- Mediated by chloroflexi
- Use organochlorine compounds as electron
acceptors - Some spp are also sulfate reducers
- Usually removes chlorines at meta and para but
not ortho positions - Several pathways identified
30
Dechlorination pathways
From Bedard, 2003
31
PCBs 47 and 51 build up as intermediates
These congeners are abundant in Aroclors
Flanked and doubly flanked meta Cls removed
PCB 51
PCB 132
Cannot be further dechlorinated by this pathway
Only unflanked para Cls remain
PCB 153
Flanked meta Cls removed
PCB 47
PCBs 47 and 51 noted as recalcitrant products by
Magar et al. (EST 2005) in Lake Hartwell
sediments
32
Further dechlorination of PCBs 47 51by a
different pathway (bacterial consortium?)
PCB 51
PCB 19
End products of dechlorination (Seen in Upper
Hudson River and elsewhere)
Unflanked para Cls removed
PCB 47
PCB 4