17'8: Colloids - PowerPoint PPT Presentation
1 / 18
Title:
17'8: Colloids
Description:
Distinct particles suspended in some medium. The Tyndall effect ... Example: Electron configuration of vanadium. V. V V 3 [Ar] 4s2 3d3 [Ar] 3d4 [Ar] 3d2 ... – PowerPoint PPT presentation
Number of Views:76
Avg rating:3.0/5.0
Title: 17'8: Colloids
1
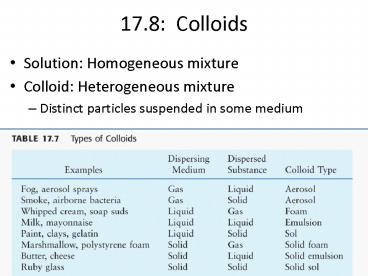
17.8 Colloids
- Solution Homogeneous mixture
- Colloid Heterogeneous mixture
- Distinct particles suspended in some medium
2
The Tyndall effect
- Collids have 1 1000 nm aggregates dispersed in
solution - These aggregates scatter light
- A solution has no light scattering
- No aggregates!
3
What stabilizes a colloid?
- Particles attract a layer of ions of the same
charge - This electrostatic repulsion helps stabilize
colloid
4
Chapter 19
- Transition Metals and Coordination Chemistry
- (Chapter 20 in 5th edition of book)
Solid K3Fe(CN)6
Metal EDTA Complex
Aqueous Co(NH3)5ClCl2
5
Transition Metals
- Useful for many things as neutral solids
- Building Materials (iron, copper, titanium,
steel) - Electrical conductivity (wires made of copper)
- In chemistry, metal ions form compounds with
nonmetals, called either - Complex ions
- Coordination complex
- Coordination compound
6
Electron Configurations
- Electron Configurations are different between
metals and metal ions - Metals as you would expect
7
Electron Configurations
- Except
- Cr Ar 4s 1 3d 5
- Cu Ar 4s 1 3d 10
- Special Stability of filled or half-filled d
orbitals
8
Metals vs. Metal Ions
- Cobalt Metal (Co0)
Cobalt Ion (Co2)
3dxz dyz dxy dz2 dx2-y2
4s
9
Metal Ions
- Transition metal ions do not have s electrons
- Example Electron configuration of vanadium
Ar 4s2 3d3
Ar 3d4
Ar 3d2
10
Coordination Compounds
Almost all metal ions found as complexes
Complex Ion
Ligand
Metal Ion (Co3)
Counter Ion, for charge balance
11
Coordination Compounds
- Metal-Ligand bond is mostly covalent
- Complex ion Counter ion bond is purely ionic
Dissociates in Solution
12
Coordination Number
- Number of bonds from metal ion to ligands
- Difficult to predict either 2, 4 or 6
13
Coordination Number
- What is the coordination number of the cobalt in
- Co(NH3)5ClCl2
- Co(NH3)5Cl 2
- Co(H2O)42
14
Ligands
- Ligand a lewis base that binds to a metal ion
- Remember
- Lewis Acid electron-pair acceptor
- Lewis Base electron-pair donor (has lone pair)
- Ligand binding results in a coordinate covalent
bond
15
Some Common Ligands
- Monodentate (one bond to metal ion)
19
16
Examples
- V(NH3)6Cl3
- Mn(H2O)4(Cl)2SO4
17
Some Common Ligands
- Bidentate Ligands (two bonds to metal ion)
- Any ligand with more than one bond called a
chelating ligand
Example Fe(en)2(NH2)2Br2
18
Some Common Ligands
- Polydentate Ligands
- Tridentate
- Tetradentate
- EDTA (ethylene diamine tetra acetate)