Minerals: Building blocks of rocks - PowerPoint PPT Presentation
1 / 42
Title:
Minerals: Building blocks of rocks
Description:
Retains all the characteristics of an element. Structure of an atom ... Amphibole: Ca2MgFeSi8O22. Olivine: Mg2Fe2SiO4. Uses: Glass, circuit boards. Carbonate group ... – PowerPoint PPT presentation
Number of Views:335
Avg rating:3.0/5.0
Title: Minerals: Building blocks of rocks
1
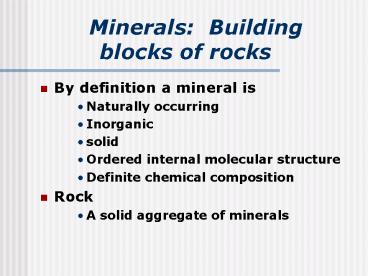
Minerals Building blocks of rocks
- By definition a mineral is
- Naturally occurring
- Inorganic
- solid
- Ordered internal molecular structure
- Definite chemical composition
- Rock
- A solid aggregate of minerals
2
(No Transcript)
3
Composition of minerals
- Elements
- Basic building blocks of minerals
- Over 100 are known (92 naturally occurring)
- Atoms
- Smallest particles of matter
- Retains all the characteristics of an element
4
Structure of an atom
- Electrons move around nucleus in shells / clouds
5
Figure 3.3
6
The Element Carbon on the Period Table
6
Atomic number
C
Why is the atomic mass of carbon not exactly
twelve?
Carbon
12.011
Atomic mass
Atomic number number of protons in an
element Atomic mass sum of the number of
protons and neutrons in an atom
Go to Section
7
Carbons Isotopes
Nonradioactive carbon-12
Nonradioactive carbon-13
Radioactive carbon-14
6 electrons 6 protons 6 neutrons
6 electrons 6 protons 8 neutrons
6 electrons 6 protons 7 neutrons
Go to Section
8
Isotopes
- Isotopes - atoms of the same element that differ
in the number of neutrons they contain - Radioactive Isotopes isotopes with unstable
nuclei that break down at a constant rate over
time - Half life time it takes for half of the atoms
to decay to the daughter product - Used to date rocks and fossils
9
Compounds and Chemical Bonds
- Compound substance formed by the chemical
combination of two or more elements in definite
proportions - Octet Rule atoms have a tendency to gain, lose,
or share electrons so they have 8 outer electrons
10
Ionic bonds Electrons are lost and gained
Sodium atom (Na)
Chlorine atom (Cl)
Sodium ion (Na)
Chloride ion (Cl-)
Protons 11 Electrons -11 Charge 0
Protons 17 Electrons -17 Charge 0
Protons 11 Electrons -10 Charge 1
Protons 17 Electrons -18 Charge -1
Transfer of electron
- Most minerals have ionic bonds
Go to Section
11
Ionic bonds Electrons are lost and gained
Sodium atom (Na)
Chlorine atom (Cl)
Sodium ion (Na)
Chloride ion (Cl-)
Protons 11 Electrons -11 Charge 0
Protons 17 Electrons -17 Charge 0
Protons 11 Electrons -10 Charge 1
Protons 17 Electrons -18 Charge -1
Transfer of electron
- Most minerals have ionic bonds
12
Halite (NaCl) An example of ionic bonding
13
Covalent bonds Electrons are shared
- Stronger than ionic bonds
- Molecule atoms are joined by covalent bonds
14
Metallic bonding
- Valence electrons are free to migrate among atoms
- Weaker and less common than other bonds
- Ex native copper
15
Minerals Building blocks of rocks
- By definition a mineral is
- Naturally occurring
- Inorganic
- solid
- Ordered internal molecular structure
- Definite chemical composition
- Rock
- A solid aggregate of minerals
16
Geometric packing of ions
- Type is determined by ionic size
Figure 3.8
17
Polymorphs
- Minerals with the same composition but different
crystalline structures - Ex Diamond and graphite
18
Physical properties Crystal form
- External expression of a minerals internal
structure - Shown only occasionally
- Often interrupted due to competition for space
and rapid loss of heat
19
Physical properties Color
- Generally unreliable for mineral identification
- Often highly variable
20
Physical properties Streak
- Color of a mineral in its powdered form
21
Physical properties Luster
- Appearance in reflected light
- Metallic
- Nonmetallic
- Subcategories are Glassy, waxy, greasy,
resinous, pearly, earthy, dull
22
Physical properties Hardness
- Resistance of a mineral to abrasion or scratching
- All minerals are compared to a standard scale
called the Mohs scale of hardness
23
Physical properties Cleavage
- Tendency to break along planes of weak bonding
- Produces flat, shiny surfaces
- Described by
- Number of planes
- Angles between planes
24
Physical properties
- Fracture
- Absence of cleavage when a mineral is broken
- Density (specific gravity)
- Weight of a mineral divided by weight of an
equal volume of water - Average value 2.7
25
Physical properties Others
- Magnetism
- Reaction to hydrochloric acid
- Malleability
- Double refraction
- Taste
- Smell
- Elasticity
26
Rock-forming minerals
- Common minerals that make up most of the rocks of
Earths crust - Composed mainly of the 8 elements that make up
over 98 of the continental crust
27
Abundance of minerals in the Earths Crust
28
Silicate group
- Silicates
- Comprise 96 of crust
- Elements Silicon-oxygen tetrahedron (SiO4)
- Fundamental building block for all silicates
- Four oxygen ions surrounding a much smaller
silicon ion - Form Crystallize from magma
29
Silicate group
- Light (felsic)
- Quartz SiO2
- Feldspar KAlSi3O8
- Dark (mafic)
- Pyroxene MgFeSiO3
- Amphibole Ca2MgFeSi8O22
- Olivine Mg2Fe2SiO4
- Uses Glass, circuit boards
30
Carbonate group
- Elements CO3
- Form Crystallize from seawater
- Examples
- Calcite Ca CO3
- Dolomite CaMg CO3
- Uses Cement, fertilizer
31
Halide group
- Elements metal and nonmetal
- Form crystallize from seawater
- Examples
- Halite NaCl
- Fluorite CaF2
- Uses salt, steel-making, fertilizer
32
Native element group
- Form often from hot water associated with
volcanoes - Examples uses
- Gold Au - circuits
- Silver Ag
- Copper Cu wires
- Diamond C cutting
- Graphite C pencil, lubricant
33
Oxide group
- Elements O
- Form chemical weathering
- Examples
- Hematite Fe2O3
- Magnetite Fe3O4
- Uses iron ore, pigment
34
Sulfate group
- Elements SO4
- Form crystallize from seawater
- Examples
- Gypsum CaSO4
- Uses Wall board, plaster
35
Sulfide group
- Elements S
- Form crystallize from hot water associated with
volcanoes - Examples
- Pyrite FeS2
- Galena PbS
- Uses metal ores
36
Important nonsilicate minerals
- Carbonates
- Primary constituents in limestone, marble, and
dolostone - Calcite (CaCO3) and dolomite CaMg(CO3)2 are the
two most important carbonate minerals - Hematite (oxide mined for iron ore)
- Halite (halide mined for salt)
- Sphalerite (sulfide mined for zinc ore)
- Native copper (native element mined for copper)
37
Rocks and the rock cycle
38
Igneous rocks
- Interlocking texture
- Igneous means from fire
- Formed when magma cools and hardens
- Magma is molten rock below ground
- Lava is molten rock on or above earths surface
- Ex Granite
39
Sedimentary rocks
- Granular or layered texture
- Natural forces (wind, waves) break down rock into
small fragments, or sediment - Sediment is carried and deposited by water, ice,
and wind - Sediment can be buried, compressed and cemented
into sedimentary rocks - Ex Sandstone
40
Metamorphic rocks
- Banded or foliated texture
- High pressure, heat, and chemical processes can
change the form of an existing rock. - The existing rock is then changed into a
metamorphic rock - Metamorphic means changed form
41
The rock cycle
- Geological processes cause rock to change from
one type to another many times - All rocks have probably passed through the rock
cycle many times during earths history
42
The rock cycle