Temperature Dependence Of Rate Coefficient - PowerPoint PPT Presentation
1 / 13
Title:
Temperature Dependence Of Rate Coefficient
Description:
For a 2nd-order reaction we can find (look up) A = 108 M 1 s 1 , ... Catalysis ... Heterogeneous Catalysis. Homogeneous: reactants and catalyst in same phase ... – PowerPoint PPT presentation
Number of Views:65
Avg rating:3.0/5.0
Title: Temperature Dependence Of Rate Coefficient
1
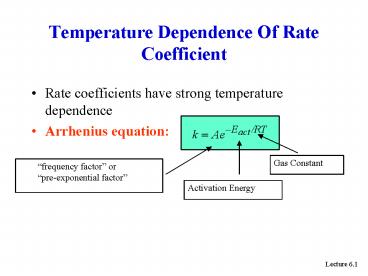
Temperature Dependence Of Rate Coefficient
- Rate coefficients have strong temperature
dependence - Arrhenius equation
2
Example
- For a 2nd-order reaction we can find (look up) A
108 M1 s1 , Eact 100 kJ mol1. - Find rate coefficients at 25 and 35º C.
- k A exp( Eact / R T )
- Exponent must be dimensionless, butunits not
consistent
Eact 100 kJ mol1 100 ? 103 J mol1.
Factor 3.7!!
Similarly, k(35º C) 1.10 ? 109 M1 s1
3
Transition State or Activated Complex
- BrCH3 OH- ? Br - CH3OH
- TRANSITION STATE or ACTIVATED COMPLEX
- geometry through which system must pass between
reactant and product - ? higher energy than reactants or products
- not a stable molecule!
4
Fig 16.19 p 690
BrCH3 OH-
Br- CH3OH
- Forward reaction is EXOTHERMIC
- Reverse reaction is ENDOTHERMIC
5
Rationalization of Arrhenius Equation
- Consider a bimolecular collision A B ? products
- must go via transition state to react, therefore
must have energy of at least Eact - fraction of collisions with energy greater than
Eact
- For gases, collision frequency ?1030 collisions
s-1 - If each collision produced reaction, reaction
rate ?106 M s-1 - Experimental gas phase reaction rates ?10-4 M s-1
- ? Only a very small fraction of collisions lead
to reaction
6
The importance of molecular orientation
Fluorine
See also textbook Figure 16.17 page 689 F2 NO2 ?
FNO2F ?
Nitrogen
Oxygen
7
Example
- Assuming the activation energies are equal,
predict which of the following reactions will
occur at a higher rate at 50º C. Explain. - NH3(g) HCl(g) ? NH4Cl(s)
- N(CH3)3(g) HCl(g) ? NH(CH3)3Cl(s)
- At the same temperature NH3 and N(CH3)3 have the
same average kinetic energy - Due to their larger mass, N(CH3)3 molecules will
be travelling more slowly - This will decrease the number of collisions per
unit time for N(CH3)3 relative to NH3 - also the higher molecular complexity of N(CH3)3
would probably reduce its orientation probability
factor
8
Catalysis
- Catalysts increase rate coefficient by providing
alternative reaction path (or mechanism) with
lower activation energy. - Catalyst does not change equilibrium, only rate
of attaining equilibrium. - Enormous practical importance all modern
chemical products. - Examples
- nitrogeneous fertilizers (N2 3H2 ? 2NH3 ) Pt
catalyst - removal of NO in vehicle exhaust Pd oxide
catalyst - cracking petroleum ? petrol, nylon,
- hydrogenating natural oils for margarine
9
Catalyst Lowers Barrier
10
Catalyst Can Significantly Change Mechanism
Fig 16.22 p 698
11
Heterogeneous Catalysis
- Homogeneous reactants and catalyst in same phase
- Heterogeneous reactants and catalyst in
different phases - eg H2 ? H H is rate limiting step in the
synthesis of ammonia N2 3H2 ? 2NH3 -
catalyzed by Pt - HH bond too strong to react with N2 Pt has
dangling bond forms weak bond with Hsweakens
HH, now H is more reactive
12
Summary
Series of plots of concentration vs time
Fig 16.12 p 686
Initial Rates
Reaction Orders
Rate Constant k and actual rate law
Integrated rate law (reaction order half-life)
Activation Energy Ea
13
Reaction Mechanisms
Determine Rate Law by Experiment