Resonance Structure - PowerPoint PPT Presentation
1 / 46
Title:
Resonance Structure
Description:
All cyclic conjugated hydrocarbons were proposed to be aromatic. ... Amorphous: small particles of graphite; charcoal, soot, coal, carbon black. ... – PowerPoint PPT presentation
Number of Views:112
Avg rating:3.0/5.0
Title: Resonance Structure
1
(No Transcript)
2
(No Transcript)
3
(No Transcript)
4
(No Transcript)
5
(No Transcript)
6
(No Transcript)
7
(No Transcript)
8
(No Transcript)
9
(No Transcript)
10
(No Transcript)
11
(No Transcript)
12
(No Transcript)
13
(No Transcript)
14
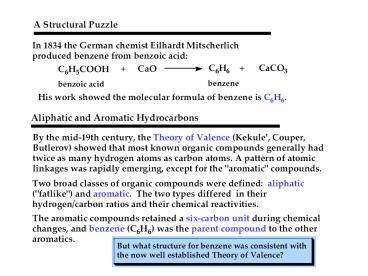
Resonance Structure
- Each sp2 hybridized C in the ring has an
unhybridized p orbital perpendicular to the ring
which overlaps around the ring.
15
(No Transcript)
16
MOs for Benzene
gt
17
Energy Diagram for Benzene
- The six electrons fill three bonding pi orbitals.
- All bonding orbitals are filled (closed shell),
an extremely stable arrangement.
18
Annulenes
- All cyclic conjugated hydrocarbons were proposed
to be aromatic. - However, cyclobutadiene is so reactive that it
dimerizes before it can be isolated. - And cyclooctatetraene adds Br2 readily.
- Look at MOs to explain aromaticity.
gt
19
Polygon Rule
- The energy diagram for an annulene has the same
shape as the cyclic compound with one vertex at
the bottom.
20
Cyclobutadiene
- Following Hunds rule, two electrons are in
separate orbitals. - This diradical would be very reactive.
21
Unstable di-radical
22
Aromatic Requirements
- Structure must be cyclic with conjugated pi
bonds. - Each atom in the ring must have an unhybridized p
orbital. - The p orbitals must overlap continuously around
the ring. (Usually planar structure) - Aromatic the compound is more stable than its
open-chain counterpart - Antiaromatic cyclic, conjugated, with
overlapping p orbitals around the ring, but the
energy of the compound is greater than its
open-chain counterpart. - Nonaromatic compounds do not have a continuous
ring of overlapping p orbitals and may be
nonplanar
23
Hückels Rule
- If the compound has a continuous ring of
overlapping p orbitals and has 4N 2 electrons,
it is aromatic. - If the compound has a continuous ring of
overlapping p orbitals and has 4N electrons, it
is antiaromatic.
gt
24
(No Transcript)
25
(No Transcript)
26
MO Derivation of Hückels Rule
- Lowest energy MO has 2 electrons.
- Each filled shell has 4 electrons.
27
(No Transcript)
28
(No Transcript)
29
(No Transcript)
30
(No Transcript)
31
(No Transcript)
32
(No Transcript)
33
Pyridine
- Heterocyclic aromatic compound.
- Nonbonding pair of electrons in sp2 orbital, so
weak base, pKb 8.8.
34
Pyrrole
- Also aromatic, but lone pair of electrons is
delocalized, so much weaker base.
35
Other Heterocyclics
gt
36
Fused Ring Hydrocarbons
- Naphthalene- 10es not 12es in two separate rings
- Anthracene
- Phenanthrene
37
Reactivity of Polynuclear Hydrocarbons
- As the number of aromatic rings increases, the
resonance energy per ring decreases, so larger
PAHs will add Br2.
(mixture of cis and trans isomers)
gt
38
Fused Heterocyclic Compounds
- Common in nature, synthesized for drugs.
gt
39
Allotropes of Carbon
- Amorphous small particles of graphite charcoal,
soot, coal, carbon black. - Diamond a lattice of tetrahedral Cs.
- Graphite layers of fused aromatic rings.
40
Some New Allotropes
- Fullerenes 5- and 6-membered rings arranged to
form a soccer ball structure. - Nanotubes half of a C60 sphere fused to a
cylinder of fused aromatic rings.
41
Common Names of Benzene Derivatives
gt
42
Disubstituted Benzenes
The prefixes ortho-, meta-, and para-
are commonly used for the 1,2-, 1,3-, and
1,4- positions, respectively.
gt
43
3 or More Substituents
Use the smallest possible numbers, but the carbon
with a functional group is 1.
gt
44
Common Names forDisubstituted Benzenes
gt
45
Phenyl and Benzyl
Phenyl indicates the benzene ring attachment.
The benzyl group has an additional carbon.
gt
46
IR and NMR Spectroscopy
- CC stretch absorption at 1600 cm-1.
- sp2 C-H stretch just above 3000 cm-1.
- 1H NMR at ?7-?8 for Hs on aromatic ring.
- 13C NMR at ?120-?150, similar to alkene carbons.
gt