Reaction kinetics: 1st order reactions - PowerPoint PPT Presentation
1 / 30
Title:
Reaction kinetics: 1st order reactions
Description:
Plotting ln[A]t against t gives a straight line with slope -k: ... Idem, from statistical mechanics (collision theory) ... Idem, from transition state theory: ... – PowerPoint PPT presentation
Number of Views:306
Avg rating:3.0/5.0
Title: Reaction kinetics: 1st order reactions
1
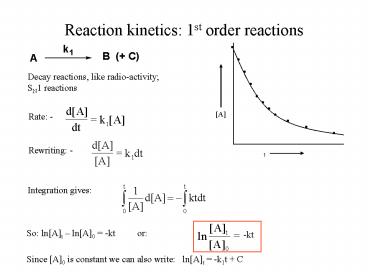
Reaction kinetics 1st order reactions
Decay reactions, like radio-activity SN1
reactions
Rate -
Rewriting -
Integration gives
So lnAt lnA0 -kt or
-kt
Since A0 is constant we can also write
lnAt -k1t C
2
Plotting lnAt against t gives a straight line
with slope -k
-kt
The halflife, t1/2, is defined as the time that
is needed to reduce the concentration of the
reactant to 50 of its original value. In formula
-kt1/2 so
3
Reaction kinetics 2nd order reactions
So -
-
When A ? B, this equation is mathematically
rather complicated.A simplification reads as
follows take P x, then A A0 x and
B B0 x The rate then becomes n
k2(A0-x)(B0-x)
so
Integration gives
Plotting
against t gives a straight line with slope
k2(B0-A0)
4
Special cases
- A0gtgtB0 (pseudo-first order kinetics)
Example
-
in which k'k2H2O
This is a pseudo-first order reaction, since
H2O is constant. The second-order rate constant
k2 can be calculated from k' and H2O. In a
dilute aqueous solution, H2O55 M.
5
Special cases
- A0 B0
Integration gives
Plotting of
against t gives k2 as the slope.
6
Reversible reactions
Take the simplest possibility On t 0 A
A0 B 0 t t A A0-x B
x k1A k-1B k1(A0 x) k-1x
k1A0 (k1 k-1)x Integration
gives (1) At equilibrium, the net
reaction rate 0, so Bt is constant (Be
xe), so k1Ae k-1Be k-1xe
7
There is an equilibrium constant so (2)
Combining eq (1) with (2) gives
This is the rate equation for a first order
process! Determination of (k1 k-1) by
plotting against t
Eq (2) gives
2 equations, 2 unknowns
Individual values of k1 and k-1 can be determined
8
Preequilibria
Very complicated kinetics, unless you assume that
AB is constant during a large part of the
reaction (steady state approach)
k1AB k-1AB k2AB (k-1 k2)AB
So the rate equation now becomes n k2AB
9
n k2AB
Two possibilities - rapid breakdown of AB,
k2gtgtk-1, so n k1AB
t
- slow breakdown of the complex k2ltltk1,k-1,
so n k2AB k2KAB
10
Interpretation of rate constantsthe Arrhenius
equation
Every reaction has to overcome an energy barrier
the transition state (TS, X). At higher
temperature, more particles are able to overcome
the energy barrier.
Arrhenius equation
Ea can be determined by measuring kobs at two
different temperatures
11
Idem, from statistical mechanics (collision
theory)
Arrhenius
P probability factor (not every collision is
effective) Z collision number (number of
collisions per second)
12
Idem, from transition state theory
or X KAB
n kX kKAB kAB, so k
kK Statistical mechanics gives us the following
relation
kB Boltzmanns constant h Plancks constant
so
13
For all equilibria we can write DG0 - RT ln K,
so for our case we get DG - RT ln
K Expressing K in terms of DG and RT gives the
following equation for k
(1)
Since DG DH - TDS, we can also write
(2)
Eq (1) and (2) are called the Eyring equations
14
The Eyring and Arrhenius equations resemble each
other
Arrhenius
so
so Ea DH RT
Eyring
In order to determine DH and DS it is easier to
differentiate ln (k/T) to 1/T
15
So, the procedure to determine activation
parameters is - determine k at different
temperatures - plotting ln(k/T) against 1/T gives
DH -
then gives DS
and when you have DH and DS, you also have DG
since DG DH-TDS
16
Interpretation of activation parameters
- DG, the Gibbs free energy of activation,
determines at which rate a certain reaction will
run at a given temperature - DH is a measure for the amount of binding
energy that is lost in the transition state
relative to the ground state (including solvent
effects) - DS is a measure for the difference in
(dis)order between the transition state and the
ground state - for monomolecular reactions DS ? 0 J/mol.K
- for a bimolecular reaction DS ltlt 0 J/mol.K(two
particles have to come together in the transition
state to form one particle, demanding a much
greater order)
17
Example
DG 62.8 kJ/mol (very fast rx) DH 33.0
kJ/mol (rel. low, compensation of
C-H bond cleavage by hydration TS) DS -100
J/mol.K (bimolecular rx)
18
Another example
DH 85 kJ/mol (relatively high no new bonds to
be formed, no compensation for the partial
cleavage of the C-C bond in the transition
state acetonitrile is aprotic, compensation of
DH by solvation will be less than in water DS
0 J/mol.K (monomolecular reaction)
19
Application of activation parameters for the
elucidation of reaction mechanisms
A DS of 12 J/mol.K was found ? monomolecular
process
A DS of -117 J/mol.K was found ? bimolecular
process rate determining step in this case is
the attack of water on the carbonyl group.
Look in your course book for the exact reaction
mechanisms!
20
Solvation (solvent effects)
Influence of solvation on the reaction rate
k(H2O) 10-7 l.mol-1.s-1 k(DMF) 10-1
l.mol-1.s-1 so DDG 30 kJ/mol
DGDMF
DGH2O
DMF
E
H2O
DGDMF lt DGH2O
reaction progress
21
What is the background of this strong solvent
effect?
In H2O there is more solvation than in DMF, due
to hydrogen bonds. Note the changes in entropy
loss of DS because of orientation of the
substrates, gain of DS because of the liberation
of water (less solvated transition state). The
balance is not easy to predict! In general, in
case of ions, the ground state is more solvated
than the transition state
TS () is hardly solvated due to the spreading of
charge. Again a strong solvent effect here
k(H2O) 7.4x10-6 s-1 k(DMF) 37 s-1
22
Solvation effects in (bio)polymers
Polymers or enzymes may have apolar pockets,
which leads to - less solvation and therefore
higher reaction rates - changes in pKas of
acidic/basic groups
PyNH
R CH3 pKa 9.7 R polymer pKa 7.7
Ka
PyNH
E.g. lysine, R-NH2 H
R-NH3
pKa (H2O) 10.4, in some enzymes pKa 7 !
23
The energy diagram
Consider the gas phase chlorination of methane
CH4 Cl
H3CHCl
rH-Cl
Reaction course is via the route of lowest energy
(mountain pass)
CH3 HCl
rC-H
24
A cross-section of this mountain landscape
gives the well-known energy diagram
H3CHCl
25
What does the transition state look like?
Hammond postulate The TS closely resembles the
species with the highest energy content
Exothermic reaction (a) has a low Ea, TS
resembles ground state Endothermic reaction (b)
has a high Ea, TS resembles the product
26
Kinetic isotope effects
Difference in effectivity of C-H and C-D bond
cleavage primary isotope effect
The background is the difference in bond
strength, caused by the difference in mass
between H and D E0 of covalent bond is given by
1/2hn 1/2hc(1/l) 1/l(C-H) ? 3000 cm-1, E0(C-H)
? 18 kJ/mol 1/l(C-D) ? 2200 cm-1, E0(C-D) ? 13
kJ/mol
27
In this case Ea D
kobs
x
? 7.5
This is the maximum primary kinetic isotope
effect at 25ºC. The isotope effect tells us
something about the transition state. For this,
we have to look at the stretching vibrations of
the C-H(D) bond
Antisymmetrical stretching vibration leads to
reaction
Symmetrical stretching vibration involvement of
H(D) depends on the structure of the transition
state
28
Symmetrical stretching vibration involvement of
H(D) depends on the structure of the transition
state
H exactly in the middle between C and Cl
symmetrical transition state, kinetic isotope
effect is maximum (7.5). H not in the middle
isotope effect lt 7.5
late TS
symmetrical TS
early TS
29
Rule of thumb kH/kD ? 4-7 bond cleavage,
symmetrical transition state kH/kD ? 1-4 bond
cleavage, asymmetrical transition state
or no bond cleavage (secondary isotope effect)
Example
Maximum isotope effect at symmetrical TS, so
when pKa(acid) pKa(HB)
30
Some more examples