Semi permeable selective membranes - PowerPoint PPT Presentation
1 / 16
Title:
Semi permeable selective membranes
Description:
The membrane allows only the smaller molecules to pass through (or, the smaller ... membrane layer (on the inside surface) supported by a highly porous substructure ... – PowerPoint PPT presentation
Number of Views:266
Avg rating:5.0/5.0
Title: Semi permeable selective membranes
1
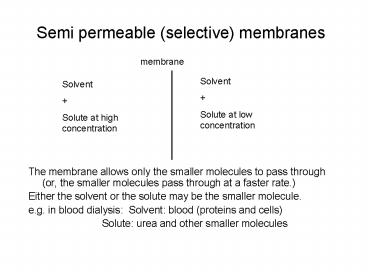
Semi permeable (selective) membranes
- membrane
- The membrane allows only the smaller molecules to
pass through (or, the smaller molecules pass
through at a faster rate.) - Either the solvent or the solute may be the
smaller molecule. - e.g. in blood dialysis Solvent blood (proteins
and cells) - Solute urea and other smaller molecules
Solvent Solute at low concentration
Solvent Solute at high concentration
2
Membrane separations
- Similarity with filtration, but different size
range. - In filtration, suspended solids are retained on
one side of the filter medium while the water
passes through. - In membrane separations we are trying to separate
material at the molecular size range.
3
(No Transcript)
4
Membrane separations
- In absorption and stripping we typically
transferred one component between the phases by
direct contact between the phases. - In adsorption, the transfer was from the fluid
phase to the surface of a solid phase, again by
direct contact. - In membrane separations we are transferring the
component through a semi-permeable barrier,
almost as in heat transfer where the fluids did
not come in direct contact but were in thermal
contact through the metal separating them.
5
Membrane separation applications
- Dialysis
- Electrodialysis
- Water purification (reverse osmosis)
- Wastewater treatment
- Pervaporation (solute goes from liquid on one
side to gas on the other)
6
Membrane materials
- Porous vs non-porous (dense polymer)
- Cellulose acetate and other polymeric materials
- Inorganic membranes
- Thickness ??m, requires structural support
- Membrane typically deposited on a thicker (and
stronger) support with high porosity and large
pore size (Fig. 26.3 MSH)
7
- Thin, dense membrane layer (on the inside
surface) supported by a highly porous substructure
8
- Blood dialysis urea and other small molecules
diffuse through membrane, proteins and blood
cells are retained
9
Mass transfer considerations
- The above formulation assumes a porous membrane
and continuity of the fluid phase. - In some cases there are distinct phase boundaries
on either side of the membrane
10
Organic-water phase boundary coinciding with
membrane surface
11
- Transport of gases through dense (nonporous)
polymer membranes occurs by a solution-diffusion
mechanism
12
Electrodialysis
13
(No Transcript)
14
- Seawater
- (35 g/L dissolved solids)
- Osmotic pressure 2740 kPa (27 atm)
15
a activity c concentration W water S salt
16
(No Transcript)