Bohrn again - PowerPoint PPT Presentation
1 / 23
Title:
Bohrn again
Description:
3. Atomic Physics. ... Atomic quantization (curve 2) Solution exists at R 2.77 a.u. ... Subject Category: Quantum physics | Atomic and molecular physics 'Bohr'n again ... – PowerPoint PPT presentation
Number of Views:24
Avg rating:3.0/5.0
Title: Bohrn again
1
Bohrn again
Marlan Scully
Texas AM University
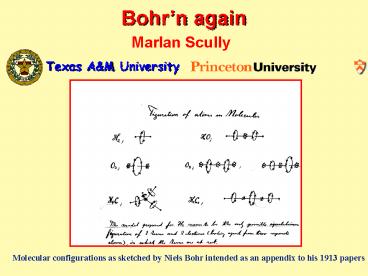
Molecular configurations as sketched by Niels
Bohr intended as an appendix to his 1913 papers
2
(No Transcript)
3
Potential energy curves of H2 molecule Solid
curves are obtained from D-scaling analysis, dots
are exact numerical answer.
4
Bohr model of H2 molecule
Energy of the system
where V is the Coulomb potential energy.
Quantization condition
5
Possible electron configurations correspond to
extrema of E
Extremum equations
For n1n21 the extremum configurations are
1)
2)
Bohr model
Exact value
6
Charge distribution in H2 molecule
7
Bohr model of atoms
8
Outer-shell electrons of Carbon form a regular
tetrahedron in Bohr model. This is similar to
bond structure of methane CH4.
9
D-scaling analysis
Examples
1. Critical phenomena are simple in 4 dimensions.
4-e dimensions critical phenomena can be
understood by perturbation theory in e.
K. Wilson and M. Fisher, Phys. Rev. Lett. 28,
240 (1972).
2. Quantum Chromodynamics is simplified if number
of colors N of quarks is large. For N3 the QCD
ground state can be obtained by 1/N expansion.
G. t Hooft, Nucl. Phys. B72, 461
(1974).
3. Atomic Physics. Schrödinger equation of an
atom becomes algebraic in the limit of infinite
dimension D ? 8. Solution in D3 can be obtained
by 1/D expansion. E. Witten, Phys.
Today 33, 38 (1980) D. Herschbach,
J. Chem. Phys. 84, 838 (1986).
4. Molecular Physics. This talk.
10
D-scaling analysis of H atom
Schrödinger equation in 3 dimensions
D-scaling transformation (D3 recovers
3-dimensional values)
In scaled variables Schrödinger equation reads
Effective potential
Limit D ? 8
Bohr model energy function
11
1/D correction
D-scaling analysis of H atom (continuation)
1/D expansion
Effective potential
Keep terms contributing in 1/D
Near the minimum
Shift in the effective potential
Harmonic oscillator
1/D correction 0
12
D-scaling analysis of H2 molecule
Full-Jacobian D-Scaling
Bohr model D-scaling
D ? 8 limit
13
D-scaling analysis of H2 molecule
D8
1
2
Ground state E(R) of H2 molecule in the limit D8
calculated in two D-scaling schemes (solid lines)
and the exact energy in three dimensions
(dots) Svidzinsky, Scully, Herschbach,
Phys. Rev. Lett. 95, 080401 (2005).
14
Bohr model of HeH
For N electrons the model reduces to finding
extrema of the energy
For HeH the three electrons cannot occupy the
same lowest level of HeH. For the configuration
n1n2n31 the right energy corresponds to a
saddle-point rather then to a global minimum.
1) Global minimum
2) Saddle-point
Bohr model
15
Bohr model of He2
Ground state
R
16
(No Transcript)
17
Constrained Bohr model
Molecular axis quantization (curve 1)
Atomic quantization (curve 2)
Solution exists at Rgt2.77 a.u.
18
Quantum mechanical constraint on electron location
In quantum mechanics the electron 1 is a cloud
with characteristic size r. Interaction potential
between the cloud and the nucleus B is F(r,R).
In the Bohr model we treat electron as a point
particle located distance r from A. Position of
the point electron on the sphere gives right
quantum mechanical answer for the particle
interaction with the nucleus B if
19
Constrained Bohr model of H2
Energy function
Constraints
20
H3 molecule (ground state)
Energy function
V Coulomb potential energy
Constraint
21
H4 molecule
Energy function
ground state
V Coulomb potential energy
22
Be2
Energy function
Constraint
where n2
23
- Nature Physics Published online 25 August 2005
- Research Highlights
- Subject Category Quantum physics Atomic and
molecular physics - 'Bohr'n again
- Andreas Trabesinger
- Abstract
- A look back at Bohr's molecular model offers a
fresh perspective on the formation of chemical
bonds between atoms in hydrogen and other
molecules. Although it is possible to model the
electronic structure of molecules - with great accuracy, such numerical methods
provide little intuitive insight into
electron-electron interactions. In - two papers, in Physical Review Letters and
Proceedings of the National Academy of Sciences,
Anatoly Svidzinsky and - colleagues 1,2 have taken a trip down memory lane
to uncover an intriguing approach to
understanding the chemical - bonds within molecules, and at the same time take
a fresh perspective on the "old quantum theory"
developed by Niels - Bohr in 1913. The famous Bohr model introduced
the quantized nature of electron orbits in
one-electron atoms, long - before wave mechanics was developed. Much later,
in the 1980s, the so-called D-scale approach
provided a quantitative - description of the two electrons surrounding a
helium nucleus, by generalizing the Schrödinger
equation to D dimensions - the situation relevant to the three-dimensional
world is deduced by interpolating between the D
1 and the D?8 limits. - However, neither approach although each
successful in its own realm has so far yielded
satisfactory results for two- - centre problems, such as the hydrogen molecule.
Svidzinsky et al. have re-examined the D-scale
approach and show how a - simple modification can fix its shortcomings 1.
Whereas the original did not even predict a bound
ground state for the - hydrogen molecule, their new version provides
quantitative values that are remarkably close to
those obtained from















![Download [PDF] Dear Jennifer, It's Me Again PowerPoint PPT Presentation](https://s3.amazonaws.com/images.powershow.com/10047088.th0.jpg?_=20240604046)
![❤[READ]❤ Find Love Again: Learn to Date Like a Goddess PowerPoint PPT Presentation](https://s3.amazonaws.com/images.powershow.com/10050326.th0.jpg?_=20240607113)
![get [PDF] Download Natasha's Kitchen: 100+ Easy Family-Favorite Recipes You'll Make Again and PowerPoint PPT Presentation](https://s3.amazonaws.com/images.powershow.com/10051551.th0.jpg?_=20240610014)





![get [PDF] DOWNLOAD Ask Me Again Tomorrow: A Life in Progress PowerPoint PPT Presentation](https://s3.amazonaws.com/images.powershow.com/10080618.th0.jpg?_=20240719047)
![READ [PDF] The Shoot-Em-Ups Ride Again PowerPoint PPT Presentation](https://s3.amazonaws.com/images.powershow.com/10094221.th0.jpg?_=20241106011)



![READ [PDF] Look Again: The Power of Noticing What Was Always There PowerPoint PPT Presentation](https://s3.amazonaws.com/images.powershow.com/10132055.th0.jpg?_=20240917081)

