Note how bonding fills all outer electron shells' - PowerPoint PPT Presentation
1 / 13
Title:
Note how bonding fills all outer electron shells'
Description:
Therefore, the covalent bonds of H2O are highly polar and so is ... Gives Water Unique Properties ... pH Matters. pH - measure of proton (hydrogen ion or H ... – PowerPoint PPT presentation
Number of Views:24
Avg rating:3.0/5.0
Title: Note how bonding fills all outer electron shells'
1
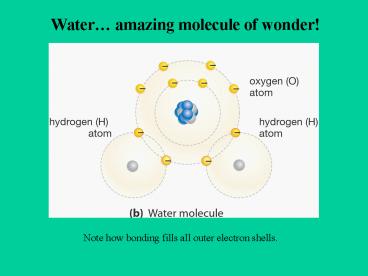
Water amazing molecule of wonder!
Note how bonding fills all outer electron shells.
2
Polar and Non-Polar Covalent Bonding
affinity for electrons.
The shared electrons spend more time around
OXYGEN relative to HYDROGEN and this covalent
bond is polar.
Therefore, the covalent bonds of H2O are highly
polar and so is the molecule.
3
Hydrogen Bonding
Oxygen is much more hungry for electrons than
hydrogen.
Bonds between oxygen and hydrogen are highly
polar.
This allows bonds to form between water
molecules! WATER IS STICKY!!!!!!!
The result is a hydrogen bond.
4
Hydrogen Bonding Gives Water Unique Properties
5
Cohesion tendency for molecules of SAME kind to
stick together
Can result in Surface Tension. Important if
youre a water bug!
Adhesion attraction that occurs between UNLIKE
molecules
Temperature moderation Water absorbs lots of
energy, but may not actually heat up. Swimming
in May or September?
Low density of ice water in ice is less dense
than liquid water (2-3 degrees is most dense for
water). Ice Floats!
6
Water Has High Surface Tension
7
Water Is Lighter as Solid than as a Liquid
8
Water Is a Wonderful Solvent
Dissolving table salt (sodium chloride)
9
Waters ability to dissolve other substances
Solution a uniform mixture of two
substances. Solvent the substance that dissolves
the other Solute the substance dissolved.
10
WATER IN ACTION
11
pH Matters
pH - measure of proton (hydrogen ion or H)
concentration .
Low pH lots of Hs High pH few Hs.
In biology, keeping H levels within a narrow
range is critically important.
12
Acids and Bases
13
- Balancing Chemical Equations
- Reactants are what you start with
- Products are the ending materials