Complex Equilibria Solubility - PowerPoint PPT Presentation
1 / 5
Title:
Complex Equilibria Solubility
Description:
7. Make chemically reasonable approximations to simplify the algebra ... 8. Solve the algebra. From Kb1: From the simplified mass balance equation: ... – PowerPoint PPT presentation
Number of Views:53
Avg rating:3.0/5.0
Title: Complex Equilibria Solubility
1
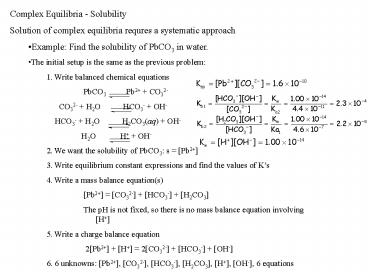
- Complex Equilibria - Solubility
- Solution of complex equilibria requres a
systematic approach - Example Find the solubility of PbCO3 in water.
- The initial setup is the same as the previous
problem - 1. Write balanced chemical equations
- PbCO3 Pb2 CO32-
- CO32- H2O HCO3- OH-
- HCO3- H2O H2CO3(aq) OH-
- H2O H OH-
- 2. We want the solubility of PbCO3 s Pb2
- 3. Write equilibrium constant expressions and
find the values of Ks - 4. Write a mass balance equation(s)
- Pb2 CO32- HCO3- H2CO3
- The pH is not fixed, so there is no mass balance
equation involving H - 5. Write a charge balance equation
- 2Pb2 H 2CO32- HCO3- OH-
- 6. 6 unknowns Pb2, CO32-, HCO3-, H2CO3,
H, OH-, 6 equations
2
- Complex Equilibria - Solubility
- Solution of complex equilibria requres a
systematic approach - Example Find the solubility of PbCO3 in water
- 7. Make chemically reasonable approximations to
simplify the algebra - Rearrange the charge balance equation
- 2Pb2 - 2CO32- - HCO3- OH- - H
solution is basic - Examine the mass balance equation
- Pb2 CO32- HCO3- H2CO3 expect
H2CO3 ltlt CO32- HCO3- - 2 unknowns have been eliminated for now
- Multiply resulting mass balance equation by 2 and
compare to charge balance equation - 2Pb2 2CO32- 2HCO3-
- 2Pb2 2CO32- HCO3- OH-
- 0 0 HCO3- - OH-
subtract - HCO3- OH-
3
- Complex Equilibria - Solubility
- Solution of complex equilibria requres a
systematic approach - Example Find the solubility of PbCO3 in water
- 8. Solve the algebra
- From Kb1
- From the simplified mass balance equation
- Pb2 CO32- HCO3-
- Solve using the method of successive
approximations or Mathcad - Pb2 3.6 x 10-5
4
- Complex Equilibria - Solubility
- Solution of complex equilibria requres a
systematic approach - Example Find the solubility of PbCO3 in water
- 9. Check assumptions
- The assumptions were
- H2CO3 ltlt CO32- HCO3- 2.2 x 10-8 ltlt
0.44 x 10-5 3.2 x 10-5 ? - H ltlt OH-
3.1 x 10-10 ltlt 3.2 x 10-5 ? - At pH8.00, Pb2 19 x 10-5 at pH9.5,
Pb23.6 x 10-5 - increasing the pH to 9.5 from 8.0 decreases the
solubility by a factor of about 5 - If CO32- did not hydrolyze
- Pb2 CO32- and
- The hydrolysis of CO32- enhances the
solubility of PbCO3 by a factor of 3!
5
- Complex Equilibria - Solubility
- Solution of complex equilibria requres a
systematic approach - Example Examine the calculation of the
solubility of AgS in pure water - Example 9-9, p. 175 FAC7