Work, Heat, and the First Law of Thermodynamics - PowerPoint PPT Presentation
1 / 17
Title:
Work, Heat, and the First Law of Thermodynamics
Description:
Gravity force, spring force) in the system ?Emech = ?K ?U = 0 ... The final point is on a lower isotherm than the initial point, that is Tf Ti. ... – PowerPoint PPT presentation
Number of Views:92
Avg rating:3.0/5.0
Title: Work, Heat, and the First Law of Thermodynamics
1
Work, Heat, and the First Law of Thermodynamics
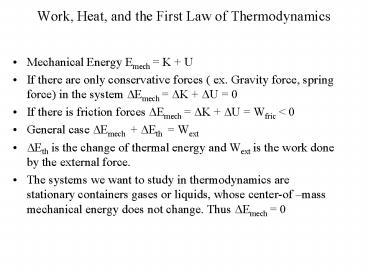
- Mechanical Energy Emech K U
- If there are only conservative forces ( ex.
Gravity force, spring force) in the system ?Emech
?K ?U 0 - If there is friction forces ?Emech ?K ?U
Wfric lt 0 - General case ?Emech ?Eth Wext
- ?Eth is the change of thermal energy and Wext is
the work done by the external force. - The systems we want to study in thermodynamics
are stationary containers gases or liquids, whose
center-of mass mechanical energy does not
change. Thus ?Emech 0
2
Thermal Energy
- Thermal energy Eth is associated with the
systems temperature. We now need to be a bit
more specific - During a phase change, from solid to liquid or
from liquid to gas, a systems thermal energy
increases but its temperature does not. - If there are no phase changes, increasing the
systems temperature increase its thermal energy. - A systems thermal energy does not change
- (?Eth 0) during an isothermal process (?T
0)
3
Work
- Work is the energy transferred between a system
and environment when a net force acts on the
system over a distance. - The sign of the work
- Work is positive when the force is in the
direction of motion - Work is negative when the force is opposite to
the motion
4
Heat
- The energy transferred in a thermal interaction
is called heat - The symbol for heat is Q
- The energy equation now becomes
- ?Esys ?Emech ?Eth Wext Q
- Quick quiz A gas cylinder and piston are covered
with heavy insulation. The piston is pushed into
the cylinder, compressing the gas. In the
process. The gas temperature - Increases
- Decreases
- Doesnt change
5
Work in Ideal-Gas Processes
- The work done on the system
- When we press the gas, the gas volume becomes
smaller, so the total work done by the
environment on the gas
6
Finding work from the P-V diagram
- W the negative of the area under the PV curve
between Vi and Vf
W lt 0
W gt 0
7
Work in some special processes
- Isochoric Process W 0
- Isobaric Process W -P?V
- Isothermal Process
Work depends on path
8
Heat and Thermal interactions
- Heat is the energy transferred during a thermal
interaction - Units of heat
- The SI unit of heat is joule.
- Historically, unit for measuring heat, is calorie
- A cal the quantity of heat needed to change the
temperature of 1 g of water by 1 oC. - 1cal 4.186 J
- 1 food calorie 1 Cal 1000 cal 1 kcal
9
Distinguish between heat, temperature, and
thermal energy
- Thermal energy is an energy of the system due to
the motion of its atoms and molecules. Thermal
energy is a state variable, it may change during
a process. The systems thermal energy continues
to exist even if the system is isolated and not
interacting thermally with its environment - Heat is energy transferred between the system and
the environment as they interact. Heat is not a
particular form of energy, nor is it a state
variable. Heat may cause the systems thermal
energy to change, but that does not mean that
heat and thermal energy are the same thing. - Temperature is a state variable, it is related to
the thermal energy per molecule. But not the same
thing.
10
The first Law of thermodynamics
- ?Esys ?Emech ?Eth W Q
- Here we assume the system mechanical energy does
not change ?Emech 0 - ?Eth W Q work on system heat to system (
first law of thermodynamics) - The first law of thermodynamics is the law of
conservation of energy. - The first law of thermodynamics doesnt tell
us anything about the value of Eth only how Eth
changes, doing 1 J of work changes the thermal
energy - by ?Eth 1 J.
- The systems thermal energy isnt the only thing
that changes. Work or heat that change the
thermal energy also change the pressure, volume,
temperature, and other state variables. The first
law tells us only about ?Eth . Other law and
relationship must be used to learn how the other
state variables change.
11
First-Law bar chart
- The isochoric process.
- The final point is on a lower isotherm than the
initial point, that is Tf lt Ti. No work is done
(W0) in this process. Heat energy was
transferred out of gas (Q lt 0) and thermal energy
of gas decreased (?E lt 0) as the temperature
fell. - ?Eth E (th f) E( th i) W Q
- The isothermal process. ?T 0
- ?Eth 0 , therefore W -Q
- The heat energy is transforred, causes the
gas to expand and do the work to environment
12
Specific Heat
- What happens to a system when you change its
thermal energy? - The temperature of the system changes
- The system undergoes a phase change, such as
melting or freezing - Specific Heat C The amount of energy that
raises the temperature of 1 Kg of - A substance by 1 K ( or 1C) is called specific
heat. In other words, the - thermal energy of the system changes by
- ?Eth Mc ?T ( Kelvin and Celsius temperature
scales have the same step size. ) - The first law of thermodynamics ?Eth W Q ,
In working with solids - And liquids, we almost always change the
temperature by heating, so W 0, - Then the heat needed to bring about a temperature
change ?T is Q Mc ?T . - Molar specific heat The amount of energy that
raises the temperature of 1 mol - of a substance by 1K. It depends on the molar
mass. - Table 17.2 (page 527) list few specific heat and
molar specific heats of solids and - Liquids.
13
Phase change and heat of transformation
- A phase change is characterized by a change in
thermal energy without change in temperature - Heat of fusion Lf the heat of transformation
for 1 Kg substance between a solid and liquid. - Heat of vaporization Lv the heat of
transformation for 1 Kg substance between a
liquid and gas. - Q M Lf melt/freeze
- Q M Lv boil/condense
- Table 17.3 list few melting/boiling temperatures
and heat of transformation. - For systems that undergo a temperature change, Q
Mc (Tf Ti). - For systems that undergo a phase change Q ML,
Supply the correct sign by observing whether
energy enters or leaves the system during the
transition.
14
Problem 45 your 300 ml cup of coffee is too hot
to drink when served at 90 C. what is the mass of
an ice cue, taken from a 20C freezer, that will
cool your coffee to a pleasant 60 C
- There are two interacting systems coffee (i.e.,
water) and ice. Changing the coffee temperature
from 90C to 60C requires four steps (1) raise
the temperature of ice from ?20C to 0C, (2)
change ice at 0C to water at 0C, (c) raise the
water temperature from 0C to 60C, and (4) lower
the coffee temperature from 90C to 60C. - Solve For the closed coffee-ice system,Q
Q(ice) Q(coffee) 0 - Q (ice) Q (ice, -20C 0) Q ( melting)
Q(water, 0 60c) - Q (coffee) m(cof)C(water) (60-90)
- Q (coffee) 0.3 Kg (4190 J?kg) ( -30) -37,710
J - Suppose the mass of ice is M
- Q (ice) 41800 M 330,000 M 251,400 M 37710
J - M 0.0605 kg 60.5 g
15
Quiz Objects A and B are brought into close
thermal contact with each other, but they are
well isolated from their surroundings. Initially
TA 0 oC and TB 10 0 oC. The specific heat of
A is less than the specific heat of B. The two
objects will soon reach a common final
temperature Tf . The final temperature is
- a. Tf gt 50 oC
- b. Tf 50 oC
- c. Tf lt 50 oC
- Answer a
- QA QB 0
- MACA(Tf 0) MBCB(Tf 100)0
- CA(Tf 0) CB(100-Tf)
16
The specific heats of gases
- The heat required to cause a specified
temperature change depends on the process by
which the gas changes
Process A and B, which start on the Ti And end on
the Tf, have the same temperature Change ?T
Tf-Ti, But different amount of Heat is required.
?T at constant volume
?T at constant pressure
Cv is the molar specific heat at
constant Volume. Cpis the molar specific heat at
constant pressure
17
Cp and Cv
- ?Eth (constant volume process) W Q 0
nCv?T nCv?T - ?Eth (constant pressure process) W Q -p?V
nCp?T - If both have the same ?T, ?Eth in tow process is
the same - -p?V nCp?T nCv?T
- Using the ideal gas law, in constant-pressure
process - p?V nR ?T
- -nR ?T nCp?T nCv?T
- Cp Cv R
- ?Eth nCv?T (any idea-gas process)






















![L 20 Thermodynamics [5] PowerPoint PPT Presentation](https://s3.amazonaws.com/images.powershow.com/A1261014311BTscN.th0.jpg?_=20160122094)







