GS 106Lecture 1 Quarks to Atoms - PowerPoint PPT Presentation
1 / 45
Title:
GS 106Lecture 1 Quarks to Atoms
Description:
In 1819, Fresnel submitted a paper on his theories to the French Academy of Sciences. ... During the conference, Fresnel was put to public ridicule for his ideas. ... – PowerPoint PPT presentation
Number of Views:28
Avg rating:3.0/5.0
Title: GS 106Lecture 1 Quarks to Atoms
1
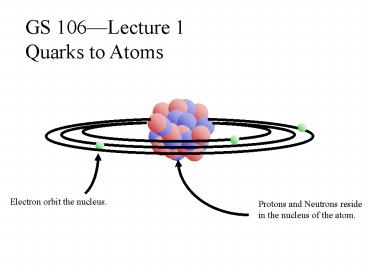
GS 106Lecture 1 Quarks to Atoms
Electron orbit the nucleus.
Protons and Neutrons reside in the nucleus of the
atom.
2
Bohrs Model of the Atom
Electron orbit the nucleus.
Protons and Neutrons reside in the nucleus of the
atom.
3
The position and number of electrons in the
orbitals of an atom determine the colors of lines
that are emitted or absorbed in atom. Too, the
number and position electrons in an atom are
primarily responsible for the chemistry of that
atom. Were going to concern ourselves now with
the nucleus of an atom.
4
Atoms with the same number of protons, but
different number of neutrons are called isotopes.
5
p
p
n
p
n
n
Different Isotopes of Hydrogen
6
Natural Radioactivity. Many naturally occurring
isotopes undergo decay. For example, Radium-226
isotopes decay into Radon.
7
Definition Half-Life Period in which one-half
of the original number of atoms undergo
radioactive decay to form a new element.
Lets say that you start with one-billion atoms
of Au-189. Gold-189 has a half-life of 30
minutes. How many gold atoms remain after 30
min, 60 min, 90 min, etc...
8
Application Carbon Dating The Carbon-14 isotope
is formed in the upper atmosphere by bombardment
of ordinary nitrogen by neutrons from cosmic
rays. The process leads to a constant ratio of
Carbon-12 to Carbon-14 atoms in the air. Living
plants (and animals that consume the plants)
incorporate Carbon into their cells as the
respire. Once the organism dies, it stops taking
in Carbon. Over time, the Carbon-14
decays. Carbon dating is very precise for objects
less than 7,000 years old and reasonably precise
for objects less than 50,000 years. For older
objects, K/Ar dating works well, for younger
objects, tritium dating works great.
9
Related Concept Doubling Time Populations have
doubling times. The current doubling rate for
the human race is 35years.
In 1895 there were 750 million people.
In 1930 there were 1.5 billion people.
In 1965 there were 3.0 billion people.
By the end of this year, there will be 6 billion
people on the planet.
10
236
Induced Nuclear Fission
11
Now we can begin to see how a chain reaction
might work.
12
(No Transcript)
13
- A nuclear reactor consists of three main
components - Fuel Rods
- Control Rods
- Moderator
14
Now all we need is a method to convert the
released energy into useable energy to power our
lights, homes, and automobiles. A nuclear
reactors energy is released in the form of heat.
We can heat up water to turn it into steam. The
steam can be used to turn turbines. Turning
turbines can generate electricity.
15
(No Transcript)
16
Another method of creating energy through a
nuclear reaction is through the fusion of two
small nuclei.
17
Nuclear Fusion Nuclear fission is the process of
splitting a big atom into smaller atoms. In the
process, small amounts of mass are turned into
energy. Meanwhile, if we take two small atoms and
smash them together, we can make a larger
atom. For example, take two Hydrogen atoms and
smash them together. In the process we end up
making a Helium atom.
18
- Remember Hydrogen comes in 3 forms
- A single proton
- A single proton and a neutron Deuteron
- A single proton and two neutrons Tritium
19
(No Transcript)
20
(No Transcript)
21
(No Transcript)
22
(No Transcript)
23
Tokamak Fusion Test Reactor
24
(No Transcript)
25
One of the last great debates before we entered
the era of modern physics concerned itself if the
nature of light. For millennia people had studied
the properties of light. S. had used the
principle of least distance to explain the
reflection of light.
?i
?r
H. had used the principle of least action to
explain refraction
26
- A question that had not been answered was
- What is light made out of?
- There were two main camps that had separate ideas
on how to go about answering this question - Light is made out of particles.
- Light is made out of waves.
27
A strong proponent of the wave theory of light
was the French engineer Augustin Fresnel. In
1819, Fresnel submitted a paper on his theories
to the French Academy of Sciences. One of the
members of the Academy did some calculations
using Fresnels approach because they theory
would lead to the absurd prediction that the
shadow of an opaque sphere should have a bright
spot at its center. During the conference,
Fresnel was put to public ridicule for his ideas.
They planned a sarcastic coup de grace by
shining a flashlight on a sphere and looking for
a central bright spot...
28
They found one.
29
Schrödingers Equation
30
For a long time people had wondering if a beam of
light consisted of particles or waves. Around
the turn of the century, it became clear that
light traveled in the form of a wave. (This is a
model that would have to be later revised a
bit). The next logical question then might be
What are electron, neutrons and protons? Are
they waves or particles?
31
At the turn of the century, the dominant model of
an atom viewed the atom as a miniature version of
a solar system
A densely packed positively charged nucleus is
orbited by a system of negatively charged
satellites.
32
It turns out, that an electron can be treated as
a wave.
33
This model of the atom nicely explains many
phenomena.
For example Line Spectra
34
As you heat up an atom, all the electrons absorb
the heat and thus gain Kinetic Energy.
This moves the electrons out to higher orbitals.
35
Over time, (usually just milliseconds or less),
the electrons fall back down into the lower
states.
As they do this, they are losing their Kinetic
Energy. The Kinetic Energy is emitted as a
photon.
36
As electrons are allowed only in certain
orbitals, they can only fall certain, well
defined distances.
This gives the us the well defined spectral lines
that we saw in lab yesterday.
37
Emission Spectra
38
Bohrs Model of the Atom explained the
observations of Line Spectra
39
Electron Source
40
Lets place a wire coil around each door. We are
going to look for induced currents in each coil.
This will tell us exactly which door each
electron passes through.
41
(No Transcript)
42
What is superfluidity? Well, its a substance
existing as a Bose-Einstein Condensate, of
course.
43
- Matter comes in two different types
- Fermions
- Bosons
- Fermions are called spin-half particles. Common
fermions are electrons and protons. - Bosons are called integer spin particles. Common
bosons are photons (light) and He-4 atoms.
44
Fermions obey something called The Pauli
Exclusion Principle. This simply means that two
fermions cant be in the same place at the same
time. Bosons dont obey the Pauli Exclusion
Principle. This means that two Bosons can be in
the same place at the same time.
http//www.colorado.edu/physics/2000/bec/
45
The 1996 Nobel Prize was given to the group who
first created a group bosons all exiting in the
place at the same time--this was call a
Bose-Einstein Condensate, or a Super-Fluid.