pH - PowerPoint PPT Presentation
1 / 14
Title:
pH
Description:
Product BA of neutralization reaction between an acid and a base. HA BOH ... energy gain once the more stable CH3COOH is formed from acetate anion and water. ... – PowerPoint PPT presentation
Number of Views:153
Avg rating:3.0/5.0
Title: pH
1
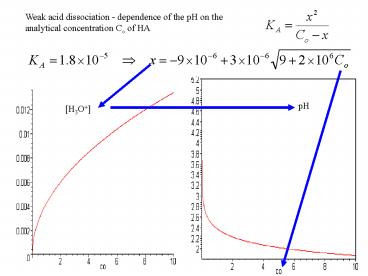
Weak acid dissociation - dependence of the pH on
the analytical concentration Co of HA
pH
H3O
2
The same for a weak base
OH-
H3O
pH
pH
pH 14 - pOH
pOH
3
Hydrolysis of a salt
What it is a salt? Product BA of neutralization
reaction between an acid and a base
HA BOH H2O BA
What is going on with the salt once we dissolve
it in a solvent (water)?
1. BA DISSOCIATES
CH3COONa CH3COO- Na
2. Resulting ions interact with the solvent
(water) molecules
Of the two above reactions the dominant one
determines the pH of the hydrolyzed salt solution.
4
Compare these equilibria products according to
the stability and energy gain if they are formed.
NaOH is strong base in the normal state this
molecule is totally dissociated. Tendency to form
NaOH product through the second reaction is
therefore minimal. Forward reaction of Na 2
H2O is against this tendency low concentration
of the product.
CH3COOH is a weak acid. Its formation via the
first reaction is thus favored by the energy gain
once the more stable CH3COOH is formed from
acetate anion and water. Water thus acts as an
acid that neutralize the base conjugated to the
acetic acid.
5
CH3COO- H2O CH3COOH OH-
If CH3COOH is formed, the other product (OH-) is
responsible for the basic pH in the water
solution of CH3COONa
Hydrolysis of a weak acid and a strong base salt
results in a basic pH of the resulting solution.
What about the opposite situation hydrolysis of
a salt of strong acid and weak base.
NH3 is a weak base and HCl is a strong acid. This
favors the first of the two hydrolysis reactions
with H3O as a product Hydrolysis of a strong
acid and a weak base salt results in an acidic pH
of the resulting solution because strong acid
molecules dissociate immediately while weak base
molecules are the stable hydrolysis product that
favors the shift of the equilibrium towards
destruction of NH3
6
BUFFERS
1.88
HA
H3O A-
Co0.5 M
xH3OHCOO-0.00989726, pH2.03
7
0.0359
H3O
Me A-
CoSalt0.5 M
Co0.5 M
xH3OHCOO-0.0001798, pH3.75
8
Approximate solution - x ltlt CoSalt
xH3OHCOO-0.0001799, pH3.74
Result is practically identical to pH3.75
9
What happens if we add 1.0 mL of 0.1 M HCl to 50
mL of water
What happens if we add 1.0 mL of 0.1 M HCl to 50
mL of buffer
Added acid is neutralized by the base present in
the buffer solution What is the base there? HCOOH
is the acid, HCOO- is the conjugated base!
Chemically HCl HCOO- --gt HCOOH Cl-
10
(No Transcript)
11
H3O
(
)
CoSalt
.00196
x
x
.00018
-
-
CoAcid
.00196
x
12
Henderson-Hasselbalch (HH) equation
13
Here comes the chemistry - KA depends on the acid
structure- we need to select the structure that
has the tendency to dissociate that corresponds
to pKA4.6 - not exactly possible, but CH3COOH is
close - pKA 4.74
Adjust pKA by selecting the proper ratio of
acid/salt
14
Buffer capacity
Adjustment of pKA is given only by the
acid/salt ratio, not by the absolute
concentrations of the two components. What is the
difference between the buffer prepared with 0.10
mol of sodium acetate and 0.140 mol of acetic
acid and the buffer prepared with 1.0 mol of
sodium acetate and 1.40 mol of acetic acid?
More concentrated buffer has larger pool of
base or acid ready to neutralize the external
addition of HA or BOH