Chapter 4: Arrangement of Electrons in Atoms - PowerPoint PPT Presentation
1 / 18
Title:
Chapter 4: Arrangement of Electrons in Atoms
Description:
X-ray (see fig. 1 pg. 98) Homework: pg. ... Pictures of Photoelectric Effect ... Draw a picture of what is happening on an atomic scale in absorption spectroscopy. ... – PowerPoint PPT presentation
Number of Views:160
Avg rating:3.0/5.0
Title: Chapter 4: Arrangement of Electrons in Atoms
1
- Chapter 4 Arrangement of Electrons in Atoms
- Properties of Light Light acts as a wave when
propagating through space and as a particle when
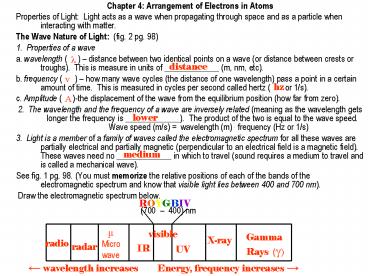
interacting with matter. - The Wave Nature of Light (fig. 2 pg. 98)
- 1. Properties of a wave
- a. wavelength ( ) distance between two
identical points on a wave (or distance between
crests or troughs). This is measure in units of
_____________ (m, nm, etc). - b. frequency ( ) how many wave cycles (the
distance of one wavelength) pass a point in a
certain amount of time. This is measured in
cycles per second called hertz ( or 1/s). - c. Amplitude ( )-the displacement of the wave
from the equilibrium position (how far from
zero). - 2. The wavelength and the frequency of a wave
are inversely related (meaning as the wavelength
gets longer the frequency is _____________). The
product of the two is equal to the wave speed.
Wave speed (m/s) wavelength (m) . frequency
(Hz or 1/s) - 3. Light is a member of a family of waves called
the electromagnetic spectrum for all these waves
are partially electrical and partially magnetic
(perpendicular to an electrical field is a
magnetic field). These waves need no
_____________ in which to travel (sound requires
a medium to travel and is called a mechanical
wave). - See fig. 1 pg. 98. (You must memorize the
relative positions of each of the bands of the
electromagnetic spectrum and know that visible
light lies between 400 and 700 nm). - Draw the electromagnetic spectrum below.
?
distance
?
hz
A
lower
medium
ROYGBIV
(700 400) nm
µ
visible
Gamma Rays (?)
X-ray
Micro wave
radio
radar
IR
UV
Energy, frequency increases ?
? wavelength increases
2
greater frequency (? indicates color)
3
EM Spectrum
HIGH ENERGY
LOW ENERGY
4
- 4. Visible, ultraviolet light, and x-rays can be
produced from electrons jumping from higher to
lower _________ levels. Elements give off a
unique color when __________ . The heat causes
electrons to jump up to higher energy levels.
Later when the electrons jump _________ to lower
energy levels, they give off light. Only certain
colors are given off for a given element (Bohr
studied hydrogen) and because of this Bohr stated
that energy levels are _____________ (meaning can
be one value or another, nothing in between).
From this, the Bohr planetary model of the atom
was created which will be discussed in more
detail later. - 5. Electromagnetic waves all travel the speed of
light (c 3.00.108 m/s). - c ??
- ? wavelength (m) ? frequency (hz)
energy
heated
down
quantized
- Practice problem
- Determine the wavelength of the light emitted by
a sodium vapor lamp if the frequency of the - radiation is 5.10.1014 hz. In what region of
the electromagnetic spectrum does this light
exist?
c ??
? 5.88.10-7 m 588.10-9 m (588 nm) Visible
light
? c / ?
? 3.00.108 m/s / 5.10.1014 hz
? 5.88.10-7 m
b. Determine the frequency of light whose
wavelength is 0.500 nm. In what region of the
electromagnetic spectrum is this radiation
located?
c ??
? c / ?
? 3.00.108 m/s / (0.500.10-9 m)
? 6.00.1017 hz X-ray (see fig. 1 pg. 98)
5
- Homework
- pg. 124 1 4 6 10 1.What is the wavelength
of light with a frequency of 6.65.1014 Hz? - 2.What is the frequency of light if its
wavelength is 695 nm? bonus 13 (c d / t)
Plancks Hypothesis 1. German scientist Max
Planck stated that energy (light) instead of
being radiated (given off) continuously, was
given off in packets or bundles of energy called
___________ . 2. Light particles (called
photons) contain specific amounts of energy. 3.
Planck came up with the idea of energy being
quantized by the analysis of a heated piece of
_____. As the iron is heated it changes in
_______ from black to red, to yellow, to white to
blue. He stated that energy changed in specific
units and was therefore ____________ (a wild idea
at the time for it clashed completely with
classical physics). (Bohr used Plancks ideas
then to create his planetary model of the atom
where electrons were quantized in orbits of fixed
energy. That led to the modern quantum
mechanical model and the idea of orbitals). 4.
Planck found a direct relationship between energy
and frequency of a photon (as frequency of a
radiant particle increases, the energy of the
photon _______________). E h ? E energy
(J) h Plancks constant (6.63.10-34
Js) Since ? c / ? E hc / ? (this is a
derived equation and will not be given on the
test) 5. Since x-rays have ________
frequencies, they have ________ energies/photon.
Since radio waves have _________ frequencies,
they have _________ energies/photon. Since gamma
rays have _________ wavelength, they have
_____________ energies/photon (so energy and
wavelength have an _____________ relationship
with each other).
quanta
iron
color
quantized
increases
high
large
low
small
short
large
inverse
6
- Planck (1900)
- Observed - emission of light from hot objects
- Concluded - energy is emitted in small, specific
amounts (quanta)
7
- Planck (1900)
vs.
8
- Practice Problems
- a. Calculate the energy of a photon whose
frequency is 5.00.1015 hz. - b. Calculate the energy of a photon whose
wavelength is 400. nm.
Eh?
E(6.63.10-34 Js)(5.00.1015hz)
E 3.32.10-18 J
c??
Ehc/?
E (6.63.10-34 Js)(3.00.108 m/s)
400..10-9 m
? c / ?
? (3.00.108 m/s) / (400..10-9 m)
E 4.97.10-19 J
? 7.5000 .1014 hz
Eh?
E(6.63.10-34 Js)(7.5000.1014hz)
E 4.97.10-19 J
9
- Homework
- pg. 124 5 11
- 1. How much energy is in a photon of light with
wavelength 580 nm? - 2. How much energy is in a photon of light with a
frequency of 5.47.1014 Hz? - 3. What is the frequency of light with energy of
4.05 x 10-19J? - 4. What is the frequency of light with wavelength
of 423 nm? - Draw a diagram of 2 waves of different frequency
and label the amplitude and wavelength on each. - Label one as high frequency and one as low
frequency. - 6. At what speed do all electromagnetic waves
travel? - 7. What scientist determined the relationship of
frequency to energy of electromagnetic radiation? - 8. Infrared radiation, uv radiation from the
sun, a green traffic light, the signal from a
radio tower, dental - x-rays, microwaves.
- arrange the radiation shown above in order of
increasing wavelength. - Arrange the radiation shown above in order of
increasing frequency. - Which order (a or b) will be correct for
increasing energy? - Bonus (must be solved as a factor-label
conversion problem for credit) - Determine the amount of time in minutes that will
be required for light to travel from Earth to
Mars? - (the distance from Earth to Mars is 1.29.105
miles)
10
- Photoelectric Effect and Atomic Spectra of
Elements - The Photoelectric Effect (see pg. 99-100)
- 1. The particle nature of light was proposed by
___________ in the 1600s, while evidence for its
wave nature was shown by ____________. The
debate as to whether light behaved as a particle
at all raged - on until the 1900s when Albert Einstein
showed that although light behaves as a _________
when traveling through space, it behaves as a
______________ when interacting with matter. - 2. Einstein showed that light behaved as a
particle when interacting with matter through his
explanation - of the photoelectric effect. The
photoelectric effect is the ejecting of _____
from an active metals surface by shining light
on the surface of the metal. It was observed
that very ________ red light would not eject
electrons from the metal surface but very ____
yellow light would eject electrons from an active
metals surface. This phenomenon could not be
explained by wave theory which states the
brighter the light is, the greater the wave
energy would be. - 3. Einstein, using Plancks hypothesis, reasoned
that each photon of the _________ frequency red
light - had a smaller energy than each photon of
energy from the yellow light (like an ant cant
kick a football off of a tee (red light) but a
football kicker can (yellow light)). - 4. The frequency of light that would just
suffice to give enough energy to _______ the
electron is called the threshold frequency. The
brighter the light shown on the metal surface
(above the threshold frequency), the _______
electrons that will be ejected (like lots of
football kickers each with their own football on
a tee). The greater the frequency of the light
(or shorter wavelength), above the threshold
frequency, the ____________ (more kinetic energy)
the electron will be ejected from the metal
surface (like an elementary football kicker (low
frequency above the threshold) compared to an NFL
kicker (higher frequency above threshold
frequency)). - Applications of Photoelectric effect (electric
eyes and photocells)
Newton
Huygens
wave
particle
e-s
bright
dim
lower
eject
more
faster
11
- Einstein (1905)
- Observed - photoelectric effect
12
- Pictures of Photoelectric Effect
- bright red light dim yellow light bright
yellow light dim blue light - (no e- ejected) (e- ejected)
(more e- ejected) (faster e- ejected)
Atomic Spectra of Elements (absorption and
emission spectroscopy) Absorption Spectroscopy
(see fig. 8a pg. 102) 1. When light is shown on
a piece of material (like a shirt), some of the
wavelengths may be __________. The absorbed
wavelengths provide an energy ________ to a
difference in energy between energy levels of
the atom absorbing the light (E h?, c ??).
What light you see emanating from the shirt is
all the light that was not absorbed (the
absorbed light warms up the shirt). 2. When
an electron absorbs a light photon it ________
into a higher energy level (here the electrons
goes from a ground state to an excited state).
3. Since elements have different arrangements
of electrons, they will have different energy
values between energy levels and thus different
wavelengths will be ____________, so different
elements are different colors.
absorbed
equal
jumps
absorbed
13
- Draw a picture of what is happening on an atomic
scale in absorption spectroscopy.
Emission Spectroscopy (fig. 5 7 pg. 101) 1. In
emission spectroscopy, gaseous atoms are placed
into an __________ state by being heated
(electricity works as well). Nanoseconds after
being excited, the electron _________ back into
the ground state where it will emit light of an
energy equal to the difference in energy between
the energy levels (this difference in energy
will determine the wavelength of the light (E
h?, c ??) 2. The light can be separated
through the use of a _________ or diffraction
grating. Draw a picture of what is happening on
the atomic scale for emission spectroscopy.
excited
relaxes
prism
Lights energy to difference in energy
between energy levels
14
- 3. Robert Bunsen observed that placing different
salts in a flame produced different colors, now - referred to the _________ test.
- 4. An elements absorption or emission spectrum
is like a _________________ that can be used to
identify the element. - Absorption spectroscopy applications Colors you
observe from materials, determination of
concentrations of solutions, identification of
elements. - Emission spectroscopy applications Lights from
incandescent bulbs, light from stars,
determination of the elemental composition of
stars (or heated gaseous elements) from their
spectral patterns, fireworks, LASERS, neon signs.
- Emission Spectroscopy Review Next Series of Slides
flame
fingerprint
15
Bohr Model
- e- exist only in orbits with specific amounts of
energy called energy levels - Therefore
- e- can only gain or lose certain amounts of
energy - only certain photons are produced
16
Line-Emission Spectrum
excited state
ENERGY IN
PHOTON OUT
ground state
17
Emission Spectrum
IR
6
- Energy of photon depends on the difference in
energy levels - Bohrs calculated energies matched the IR,
visible, and UV lines for the H atom
5
4
3
2
1
18
- Each element has a unique bright-line emission
spectrum. - Atomic Fingerprint
Helium