Coherent Radiation - PowerPoint PPT Presentation
1 / 23
Title:
Coherent Radiation
Description:
wave-particle duality. 4. Interaction between electromagnetic radiation ... The de Broglie's Particle Wave ... and matter a dual wave-particle nature. l = h / p ... – PowerPoint PPT presentation
Number of Views:118
Avg rating:3.0/5.0
Title: Coherent Radiation
1
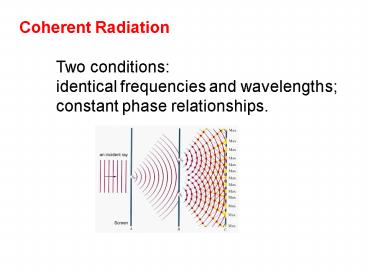
Coherent Radiation Two conditions identical
frequencies and wavelengths constant phase
relationships.
2
(No Transcript)
3
Understanding the nature of light
1. Light is composed of particles
2. Light is wave
a. General concepts of Wave
(wavelength, frequency, velocity, amplitude)
b. Properties of Wave
I. Diffraction Coherent Radiation
II. Transmission Dispersion
III. Refraction Snells Law
3. Black Body Radiation and photoelectric effect
wave-particle duality
4. Interaction between electromagnetic radiation
and matter for spectroscopy scattering,
absorption, and emission
4
Transmission of Radiation It is observed
experimentally that the rate at which radiation
is propagated through a transparent substance
is less than its velocity in a vacuum and depends
upon the kinds and concentrations of atoms, ions,
or molecules in the medium. It follows from
these observations that the radiation must
interact in some way with the matter. Because a
frequency change is not observed, however, the
interaction cannot involve a permanent energy
transfer. The refractive index of a medium is
one measure of its interaction with radiation
and is defined by
vi velocity of the radiation in the medium
5
Effect of Change in Medium on Radiation frequency
does not change, but the wavelength changes.
l1/l2 v2 / v1 Refractive index h a
measure of the medium's interaction with
radiation. hi c/vi Most liquids 1.3 to
1.89 Solids 1.3 to 2.5
6
The Effect of medium change on a monochromatic
beam of radiationlight velocity can be changed?
l1/l2 h2 / h1 500nm/300nm h2 / h1 h1 1
h2 1.67
7
Dispersion Since the velocity of radiation in
matter is wavelength dependent and since c in
Equation 6-11 is independent of this parameter,
the refractive index of a substance must also
change with wavelength. Dispersion in a
transparent material is its variation in
refractive index as a function of wavelength or
frequency. Dispersion plots exhibit two types of
regions. In the normal dispersion region, there
is a gradual increase in refractive index with
increasing frequency (or decreasing wavelength)
Dispersion is important when choosing materials
for the optical components of instruments.
8
Figure 6-9 A typical dispersion curve.
Anomalous dispersion is the change in refractive
index of a substance in a wavelength region where
absorption is occurring
9
Understanding the nature of light
1. Light is composed of particles
2. Light is wave
a. General concepts of Wave
(wavelength, frequency, velocity, amplitude)
b. Properties of Wave
I. Diffraction Coherent Radiation
II. Transmission Dispersion
III. Refraction and reflection
3. Black Body Radiation and photoelectric effect
wave-particle duality
4. Interaction between electromagnetic radiation
and matter for spectroscopy scattering,
absorption, and emission
10
- Refraction
11
Refraction of Radiation When radiation passes at
an angle through the interface between two
transparent media that have different densities,
an abrupt change in direction, or refraction, of
the beam is observed as a consequence of a
difference in velocity of the radiation in the
two media. When the beam passes from a less
dense to a more dense environment, as in Figure
6-10, the bending is toward the normal to the
interface. Bending away from the normal occurs
when the beam passes from a more dense to a less
dense medium.
12
Figure 6-10 Refraction of light in passing from
a less dense medium M1 into a more dense medium
M2, where its velocity is lower.
13
Snells Law
14
Snell's law sin q1 / sin q2 h2 /
h1 v 1 / v2 In vacuum, v becomes equal to c
and n is 1. h2 (sin q1 ) vacuum / sin q2
15
- Reflection
16
(No Transcript)
17
(No Transcript)
18
Understanding the nature of light
1. Light is composed of particles
2. Light is wave
a. General concepts of Wave
(wavelength, frequency, velocity, amplitude)
b. Properties of Wave
I. Diffraction Coherent Radiation
II. Transmission Dispersion
III. Refraction and reflection
3. Black Body Radiation and photoelectric effect
wave-particle duality
4. Interaction between electromagnetic radiation
and matter for spectroscopy scattering,
absorption, and emission
19
Photoelectric Effect
Einstein (1921)
Photon (1905) photons have energy that only
depends on their frequency times a constant h,
called Plancks constant, 6.626 x 10-34 Js.
E hn
c
Light is A Particle Again!
Work function
20
The de Broglies Particle Wave
- In 1923, Louis de Broglie suggested that matter,
as well as light, might have wave properties!!! - This would give both light and matter a dual
wave-particle nature.
l h / p l wavelength, h Planks constant
and p momentum
de Broglie (1929)
21
Understanding the nature of light
1. Light is composed of particles
2. Light is wave
a. General concepts of Wave
(wavelength, frequency, velocity, amplitude)
b. Properties of Wave
I. Diffraction Coherent Radiation
II. Transmission Dispersion
III. Refraction Snells Law
3. Black Body Radiation and photoelectric effect
wave-particle duality
4. Interaction between electromagnetic radiation
and matter for spectroscopy scattering,
absorption, and emission
22
Understanding the nature of light
1. Light is composed of particles
2. Light is wave
a. General concepts of Wave
(wavelength, frequency, velocity, amplitude)
b. Properties of Wave
I. Diffraction Coherent Radiation
II. Transmission Dispersion
III. Refraction Snells Law
3. Black Body Radiation and photoelectric effect
wave-particle duality
4. Interaction between electromagnetic radiation
and matter for spectroscopy scattering,
absorption, and emission
23
Preview Chapter 7 p. 143-152 154-161
167-172 179-181
Homework in Chapter 7 2, 8, 9, 11, 12, 15,