Summary statistics for particle size distribution - PowerPoint PPT Presentation
1 / 35
Title:
Summary statistics for particle size distribution
Description:
Particle size distribution is plotted as the mass which is ... Summary statistics for particle size distribution. d50, d10, d80 etc. ... of size classes? ... – PowerPoint PPT presentation
Number of Views:824
Avg rating:3.0/5.0
Title: Summary statistics for particle size distribution
1
Summary statistics for particle size distribution
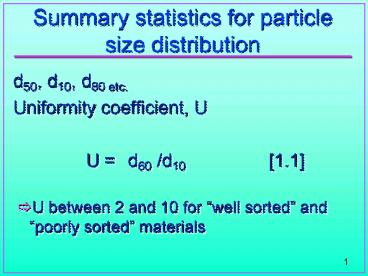
- d50, d10, d80 etc.
- Uniformity coefficient, U
- U d60 /d10 1.1
- U between 2 and 10 for well sorted and poorly
sorted materials
2
Dependence of bulk density on particle size
distribution
- Uniform particle size distribution gives low
packing density - increasing the range of particle sizes gives
rise to greater bulk density.
3
(No Transcript)
4
What are is the basis of size classes?
- Clay wont settle (lt2m doesnt feel gritty
between your teeth). - Silt settles freely, but cannot be
discriminated by eye (isnt slippery between your
fingers doesnt make strong ribbons goes
through a number 300 sieve 2mltsiltlt0.05mm). - Sand you can see (gt0.05 mm), but is smaller
than pebbles (lt2mm).
5
Systems of soil textural classification
- (The USDA is standard in the US)
6
Sand, Silt, Clay Textural Triangle
- Standard textural triangle for mixed grain-size
materials
Clay axis
Silt axis
Sand axis
7
Soil Classification
- Based on present features and formative
processes - Soil is geologic material which has been altered
by weathering an biological activity. Typically
extends 1-2 meters deep below soil is parent
material - Soil development makes sequence of bands, or
horizons.
8
Eluvial processes
- Clay is carried with water in eluviation and
deposited in illuviation in sheets (lamellae)
making an argillic horizon. - Soluble minerals may be carried upward through a
soil profile driven by evaporation giving rise to
concentrated bands of minerals at particular
elevations.
9
Vertical Variations in Soils
- Banding also arises from the depositional
processes (parent material). - The scale of variation shorter in the vertical
than horizontal. - Layers may be very distinct, or almost
indistinguishable.
10
System of designations
- Three symbol designation e.g. Ap1
- A here is what is referred to as the
designation of master horizon - There are six master horizon designations O, A,
E, B, C, and R.
11
Master Horizon Designations
- O dominated by organic matter.
- A first mineral horizon in a soil with either
enriched humic material or having properties
altered by agricultural activities (e.g.,
plowing, grazing). - E loss of a combination of clay, iron and
aluminum only resistant materials. Lighter in
color than the A horizon above it (due to a
paucity of coatings of organic matter and iron
oxides).
12
Master Horizon Designations (cont.)
- B below A or E, enriched in colorants (iron and
clays), or having significant block structure. - C soil material which is not bedrock, but shows
little evidence of alteration from the parent
material. - R too tough to penetrate with hand operated
equipment. - For complete definitions, see the SCS Soil
Taxonomy (Soil Conservation Service, 1994).
13
Master Horizon Designations (cont.)
- Major designations may be combined as either AB
or A/B if the horizon has some properties of the
second designation
14
Subordinate classifications
- Lower case letter indicates master horizon
features. - There are 22. e.g.
- k accumulation of carbonates
- p plowing
- n accumulation of sodium
- May be used in multiple
15
Final notes on designations
- Arabic numerals allow description of sequences
with the same master, but with differing
subordinate (e.g., Bk1 followed by Bn2). - Whenever a horizon is designated, its vertical
extent must also be reported.
16
Color and Structure tell genetic and
biogeochemical history
- Dark colors are indicative of high organic
content - Grayish coloration indicates reducing (oxygen
stripping) conditions - Reddish color indicates oxidizing (oxygen
supplying) conditions. - Relates closely to hydraulic conditions of site
- Often of greater use than a slew of lab analysis
of soil cores.
17
Quantification of Color
- Munsell Color chart by hue, value and chroma
summarized in an alpha-numerical coding
shorthand. - Pattern of coloration is informative. Mottling,
where color varies between grayish to reddish
over a few cm, most important. - Intermittent saturation oxidizing then reducing
- Precise terminology for mottle description (e.g.,
Vepraskas, M.J. 1992).
18
Structure
- Must identify the smallest repeated element which
makes up the soil ped Include details of the
size, strength, shape, and distinctness of the
constituent peds.
19
Climate
- Six major climatic categories employed in soil
classification useful in groundwater recharge
and vadose zone transport. - Aquic precipitation always exceeds
evapotransiration (ET), yielding continuous net
percolation. - Xeric recharge occurs during the wet cool
season, while the soil profile is depleted of
water in the hot season. - Identifying the seasonality of the local water
balance is fundamental to understanding the
vadose zone hydrology.
20
- Six categories of climates
21
High Points of Clay Mineralogy
- General
- Clay constituents dominate hydraulic chemical
behavior - Two basic building blocks of clays
- silica centered tetrahedra
- variously centered octahedra
22
Basic Formations
- chain structures (e.g., asbestos)
- amorphous structures (glasses)
- sheet structure (phyllosilicates clay!)
http//whyfiles.org/coolimages/images/csi/asbesto
s.jpg
http//usgsprobe.cr.usgs.gov/gpm/dickite.gif
23
Unit-cells octa- and tetrahedral units
www.georgehart.com/virtual-polyhedra/ dice.html
http//www.pssc.ttu.edu/pss2330/images/uday15_1_3.
gifhttp//www.pssc.ttu.edu/pss2330/images/uday15_
1.gif
24
Isomorphic Substitution
- Silica tetrahedron four oxygen surrounding one
silica atom - Space filled by the silica can accommodate atoms
up to 0.414 times O2 radius (5.8 x 10-9 m)
includes silica and aluminum. - Balanced charge if the central atom has charge
4, negative charge if the central atom has a
less positive charge (oxygen is shared by two
tetrahedra in crystal so contributes -1 to each
cell). - Same for the octahedra 0.732 times O2 radius
(1.02 x 10-8 m) iron, magnesium, aluminum,
manganese, titanium, sodium or calcium, (sodium
and calcium generate cubic lattice rather than
octahedra)
25
Ionic radii dictate isomorphic substitution
Fit
into
Tetrahedron
(radius lt0.41
t
imes that of
oxygen
Fit
into
Octahedron
(radius lt0.732
Na
0.097
0.693
2
Ca
0.099
0.707
t
imes that of
K
0.133
0.950
oxygen)
2
Ba
0.13
4
0.957
Rb
0.147
1.050
26
Surface Functional Groups
- Clay minerals surfaces made up of hexagonal rings
of tetrahedra or octahedra. - The group of atoms in these rings act as a
delocalized source of negative charge surface
functional group (a.k.a. SFG). - Cations attracted to center of SFGs above
surface of the sheet. - Some (e.g., K and NH4) dehydrated and attached
to the SFG inner sphere complex with the SFG - Cations bound to the SFG by water outer sphere
complex - Inner and outer sphere ion/clay complexes are the
Stern layer.
27
Details of Stearn Layer
http//www.ornl.gov/ORNLReview/v34_2_01/p24a.jpg
- Anions will be repelled from clay surfaces.
- Zig-zag negative and positively charged elements
in clay generates dipole moment attracting
charged particles. - Diffuse attraction results in increased ionic
concentration Gouy layer (Gouy, 1910). - Dipole-dipole attraction also holds water to the
clay surfaces, in addition to osmotic force from
cation concentration near the clay surfaces.
28
Hydration of Cations
http//www-sst.unil.ch/perso_pages/Bernhard_homepa
ge/On20line20publications/Image31.gif
29
Cation Exchange
- The degree to which soil cations may be swapped
for other cations is quantified as the cation
exchange capacity (CEC) which is measured as - CEC cmol of positive charge/kgcmol() is
equal to 10 Milliequivilents (meq) - 1 CEC 1 meq per 100 grams of soil.
- Typical values of CEC are less than 10 for
Kaolinite, between 15 and 40 for illite, and
between 80 and 150 for montmorilonite.
30
Swelling of Clays
31
Distinguishing features between clays
- Order of layering of tetra and octa sheets
- Isomorphic substitutions
- Cations which are bound to the surface
functional groups
32
Examples Kaolinite
- 11 alternating octatetra sheets
- Little isomorphic substitution. Thus...
- Very stable thicker stacks
- Relatively low surface area 7-30 m2/gr
- Do not swell much
http//www.arenisca.com/kaol-2.gif
33
Examples Montmorilonite (smectite family)
- The most common smectite is Montmorillinite,
with general formula - (½Ca,Na)(Al,Mg,Fe)4(Si,Al)8O20(OH)4.nH2O
- 21 octa sandwiched in 2 tetra sheets.
- Lots of isomorphic substitutionMg2, Fe2,
Fe3 for Al3 in octa. Since the octa is
between tetras, cations in outer sphere
complexes with hydrated SFGs. Thus - High surface area (600-800 m2/gr)
- Lots of swelling
- Big CEC.
http//www.dc.peachnet.edu/janderso/acres/Sum99/t
alksan/img018.gif
34
Examples Illite
http//www.curtin.edu.au/curtin/centre/cems/report
_2000/images/41_7.jpg
- 21 octa sandwiched in 2 tetra sheets.
- Lots of isomorphic substitutionAl3 for the
Si4 in the tetra. Generates charged SFGs
binding potassium ionically between the
successive 21 units. Thus - Moderate surface area 65-120 m2/g)
- Little swelling
- moderate CEC.
http//www.glossary.oilfield.slb.com/Files/thumb_O
GL98084.jpg
35
Summary of Clays
- Clays are 10s atomic radii thick and thousands
of atomic radii in horizontal extent - high surface to weight area plate structure.
- Hold both water and cations
- Highly reactive.
- Swell wetted state due to hydration.
- Dissociate if cations which glue layers together
are depleted - Paths tortuous high resistance to flow of water
impermeableCareful in the vadose zone
shrinkage voids