BODs - PowerPoint PPT Presentation
1 / 30
Title: BODs
1
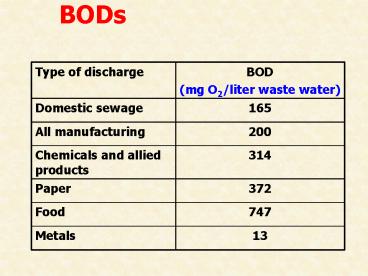
??? BODs ????????????????????????
2
????????????????????????????????????????(Decompos
ition products of organic compounds)
3
?????????????????????????????????????
4
- ???????????????????????????????
- Water and Sewage Treatment
4
5
- ????????????????????????? (water treatment)
- ???????????????????????????????????
- ?????????????????????????
6
Water treatment for domestic and commercial uses
7
Primary water treatment
- Primary treatment Remove solids by screening and
settling - The sewage is passed through a screen to remove
large pieces of debris (e.g. sticks, stones,
rags, and plastic bags). - Next, the sewage enters a grit chamber, where the
water flow is slowed just enough to allow coarse
sand and gravel to settle out on the bottom. - Water then enters the sedimentation tank, its
flow rate is further decreased to permit
suspended solids to settle out as raw sludge.
8
Primary water treatment
- Ca(OH)2 and Al2 (SO4)3 are often added to speed
up the sedimentation process. - 3 Ca(OH)2 Al2(SO4)3 ? 2 Al(OH)3 3 CaSO4
- Al(OH)3 is a gelatinous precipitation that
settles out slowly, carrying suspended material
and bacteria with it. - Oily material floats to the surface and is
skimmed off.
9
- The grit is collected and disposed in landfill.
- The raw sludge
- Old way incinerated, disposed in landfill or
dumped at sea. - New way composted to produce a nutrient rich
bacteria-free material for use as fertilizer.
10
Primary treatment
11
In older sewage-treatment plants, the water after
primary treatment is often chlorinated to kill
pathogens and then discharged into a natural
waterway. The discharged water at this stage
still contains a large amount of oxygen-consuming
wastes, which may deplete dissolved oxygen in the
water way and cause eutrophication.
is water pollution caused by excessive plant
nutrients
"blooms"
12
(No Transcript)
13
Secondary treatment (biological treatment)
- Use bacteria to break down organic compounds to
CO2. - A mixture of organisms termed activated sludge
is added to the sewage effluent. - Air or oxygen is vigorously bubbled through pipes
into the effluent.
14
(No Transcript)
15
Secondary treatment
- The aerobic bacteria digest the organic material
and break it down into CO2 and water. - The bacteria and any remaining undecomposed
material are returned to the aeration tank and
reused.
16
Activated sludge process
17
Secondary treatment of municipal wastewater
18
Most municipal plants chlorinated the water after
secondary treatment and then release it into
waterways. The discharged water at this stage has
90 of the original organic matter removed, but
over 50 of N, P species remains, and metal ions
and many synthetic organic compounds are
incompletely removed.
19
Tertiary treatment (Advanced waste treatment)
includes a variety of processes performed on the
effluent from secondary waste treatment.
- Remove N and P nutrients.
- P removal by precipitation with lime
- 3 PO43- CaO (lime) ? Ca5(PO4)3(OH)
- Phosphate can also be removed by microorganisms
that absorb phosphate.
20
- Remove N and P nutrients.
- NH4 removal by ammonia stripping.
- NH4 OH- NH3 H2O (Excess OH- from lime)
- Alternative NH4 removal nitrifying bacteria
convert NH4 to NO3- followed by denitrifying
bacteria to convert NO3- to N2. - Remove organics through filtration by activated
carbon
21
- Tertiary treatment
22
Sludge treatment The sludge collected from
primary and secondary clarifiers is treated and
dewatered through a series of steps, i.e.,
anaerobic sludge tanks, sludge holding tank,
centrifuge and belt filter press.
A. From tertiary treatmentB. Gas tankC. Heat
exchangerD. DigesterE. Sludge holding tankF.
Sludge level sensorG. Supernatant to
headworksH. Sludge polymer mixerI. Polymer feed
systemJ. CentrifugeK. To load worksL. Belt
press dewateringM. Dewatered sludge cake to
landfill
23
Tertiary treatment of municipal wastewater
Fe salt PO43-
Denitrification methanolbacteria (NO3-NH3-gtN2)
Nitrification bacteria (NH3O2-gtNO3-)
24
Add water to remove any remaining solids,
principal same as the Primary Settling Tanks.
The only difference is that the tanks in the
Tertiary Treatment process are rectangular and
do not have skimmers and sweepers.
all of the remaining solids are removed, the
water is sent to holding bays that simply hold
the water before it is filtered. It is common to
find hundreds of fish living and thriving in
this holding bay
25
Performance of primary and secondary stages of
sewage treatment
Source American Chemical Society
26
- Sludge is an excellent fertilizer in principle
rich in organic material and nutrients. - Sludge often contains toxic metal species, which
restricts the application of sludge to cropland. - Sludge could be converted to methane by anaerobic
bacteria, but this option suffers poor economics. - Sludge can be a low-quality fuel for generating
electricity.
sludge
27
Disinfection
- Common disinfectants Chlorine, chlorine dioxide,
and ozone. - Disinfectants kill microorganisms by oxidizing
vital molecules (often with unsaturated carbon
bond) in them. - Cl2 H2O HOCl H Cl-
Hypochlorous acid Active disinfection component
28
Pros and cons of various disinfectants
- Cl2
- Cl2 is effective and relatively cheap.
- HOCl can act as a chlorinating agent to produce a
variety of chlorinated organic compounds (for
example, CHCl3). - Many of the Cl-containing organics are toxic and
non-biodegradable. Some (e.g. CH2Cl2, CHCl3,
C2HCl3) are suspected carcinogens. - O3 and ClO2
- More expensive than Cl2.
- Need to be generated on-site ? add on to the
capital cost. - Fast-acting and rapidly decomposed. (Persistence
of disinfectants allows disinfect water where
leakage through old pipes occur.)
29
Generation of ClO2 and O3
- ClO2
- 2 NaClO2 (s) Cl2 (g) 2ClO2 (g) 2 NaCl (s)
- Sodium hypochlorite
- O3
- Subject pressurized air to an electric discharge
of 20,000v.
30
Study questions
- What does primary, secondary, and tertiary water
treatment achieve? How does each water treatment
step achieve its goal(s)? - What chemicals are often used for disinfection?
What is the mechanism of disinfection?