Fig. 3.11 - PowerPoint PPT Presentation
1 / 88
Title:
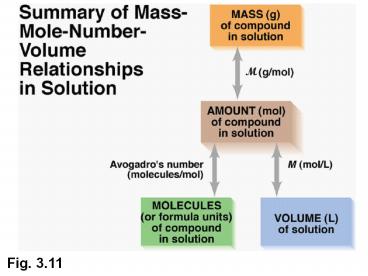
Fig. 3.11
Description:
Fig. 3.11 Reactions Involving Ions: Molecular vs. Ionic Equations Chemical Reaction can be expressed by: Molecular Equation (balanced chemical equation) Complete ... – PowerPoint PPT presentation
Number of Views:78
Avg rating:3.0/5.0
Title: Fig. 3.11
1
Fig. 3.11
2
- 3 general classes of chemical reactions
- Precipitation reactions
- Ex geology, heavy metal analysis
- Solid formation from ionic compounds
- 2) Acid/base reactions
- Ex many biochemical reactions
- Proton transfer in polar covalent compounds
- 3) Oxidation/Reduction (redox) reactions
- Ex batteries, metabolic energy production
- Electron transfer in ionic molecular compounds
3
Classifying Chemical Reactions
- Similarities in written equations
- Combination reactions (CR)
- often redox
- Decomposition reactions (DR)
- often redox
- Single replacement (SR)
- often redox
- Double displacement (DD)
- Acid/base, precipitation, redox
Lab
4
Reactions Involving IonsMolecular vs. Ionic
Equations
- Chemical Reaction can be expressed by
- Molecular Equation (balanced chemical equation)
- Complete Ionic Equation (showing all ions in
reaction) - Net Ionic Equation (showing only those ions
directly involved in reaction) - Consider
- Copper (III) sulfate reacts with sodium hydroxide
to form copper (III) hydroxide and sodium sulfate
(all in water).
- Express reaction in molecular, complete ionic,
- and net ionic equations
5
(No Transcript)
6
The Solubility of Ionic Compounds in Water
The solubility of ionic compounds in water
depends upon the relative strengths of the
electrostatic forces between ions in the ionic
compound and the attractive forces between the
ions and water molecules in the solvent. There
is a tremendous range in the solubility of ionic
compounds in water! The solubility of so called
insoluble compounds may be several orders of
magnitude less than ones that are
called soluble in water, for example
Solubility of NaCl in water at 20oC 365
g/L Solubility of MgCl2 in water at 20oC 542.5
g/L Solubility of AlCl3 in water at 20oC 699
g/L Solubility of PbCl2 in water at 20oC 9.9
g/L Solubility of AgCl in water at 20oC 0.009
g/L Solubility of CuCl in water at 20oC 0.0062
g/L
7
Precipitation Reactions Will a Precipitate Form?
If we add a solution containing potassium
chloride to a solution containing ammonium
nitrate, will we get a precipitate?
KCl(aq) NH4NO3 (aq) K(aq)
Cl-(aq) NH4(aq) NO3-(aq)
By exchanging cations and anions we see that we
could have potassium chloride and ammonium
nitrate, or potassium nitrate and
ammonium chloride. In looking at the solubility
table it shows all possible products as soluble,
so there is no net reaction!
KCl(aq) NH4NO3 (aq) No Reaction!
If we mix a solution of sodium sulfate with a
solution of barium nitrate, will we get a
precipitate? From the solubility table it shows
that barium sulfate is insoluble, therefore we
will get a precipitate!
Na2SO4 (aq) Ba(NO3)2 (aq)
BaSO4 (s) 2 NaNO3 (aq)
8
Solubility
- Soluble ability to dissolve in a liquid
- Insoluble inability to dissolve in a liquid
- Not all Ionic Compounds are water soluble
- Not all molecular compounds are insoluble!
9
8 Simple Rules For Common Ionic Compounds
Predicting Precipitation
10
- Molecular Equation
- A chemical equation in which the reactants and
products are written as if they were molecular
substances, even though they may actually exist
in solution as ions. - State symbols are include (s), (l), (g), (aq).
- For example
- AgNO3(aq) NaCl(aq) ? AgCl(s) NaNO3(aq)
- Although AgNO3, NaCl, and NaNO3 exist as ions in
aqueous solutions, they are written as compounds
in the molecular equation.
11
- Complete Ionic Equation
- A chemical equation in which strong electrolytes
are written as separate ions in the solution.
Other reactants and products are written in
molecular form. State symbols are included (s),
(l), (g), (aq). - For example
- AgNO3(aq) NaCl(aq) ? AgCl(s) NaNO3(aq)
- In ionic form
- Ag(aq) NO3-(aq) Na(aq)Cl-(aq) ?
- AgCl(s) Na(aq) NO3-(aq)
12
- Spectator Ion
- An ion in an ionic equation that does not take
part in the reaction. It appears as both a
reactant and a product.
13
- Net Ionic Equation
- A chemical equation in which spectator ions are
omitted. It shows the reaction that actually
occurs at the ionic level. - For example
- Ag(aq) NO3-(aq) Na(aq) Cl-(aq) ?
- AgCl(s) Na(aq) NO3-(aq)
- In net ionic form
- Ag(aq) Cl-(aq) ? AgCl(s)
14
(No Transcript)
15
Figure 4.6 Reaction of magnesium chloride and
silver nitrate. Photo courtesy of American Color.
Write molecular and ionic equations for this
reaction.
Ionic equation Ag(aq) Cl-(aq) ?AgCl(s)
16
- Decide whether the following reaction occurs. If
it does, write the molecular, ionic, and net
ionic equations. - KBr MgSO4 ?
- Determine the product formulas
- K and SO42- make K2SO4
- Mg2 and Br- make MgBr2
- Determine whether the products are soluble
- K2SO4 is soluble
- MgBr2 is soluble
- KBr MgSO4 ? no reaction
17
- Decide whether the following reaction occurs. If
it does, write the molecular, ionic, and net
ionic equations. - NaOH MgCl2 ?
- Determine the product formulas
- Na and Cl- make NaCl
- Mg2 and OH- make Mg(OH)2
- Determine whether the products are soluble
- NaCl is soluble
- Mg(OH)2 is insoluble
18
- Molecular Equation
- (Balance the reaction and include state symbols)
- 2NaOH(aq) MgCl2(aq) ?
- 2NaCl(aq) Mg(OH)2(s)
- Ionic Equation
- 2Na(aq) 2OH-(aq) Mg2(aq) 2Cl-(aq) ?
- 2Na(aq) 2Cl-(aq) Mg(OH)2(s)
- Net Ionic Equation
- 2OH-(aq) Mg2(aq) ? Mg(OH)2(s)
19
- Decide whether the following reaction occurs. If
it does, write the molecular, ionic, and net
ionic equations. - K3PO4 CaCl2 ?
- Determine the product formulas
- K and Cl- make KCl
- Ca2 and PO43- make Ca3(PO4)2
- Determine whether the products are soluble
- KCl is soluble
- Ca3(PO4)2 is insoluble
20
- Molecular Equation
- (Balance the reaction and include state symbols)
- 2K3PO4(aq) 3CaCl2(aq) ?
- 6KCl(aq) Ca3(PO4)2(s)
- Ionic Equation
- 6K(aq) 2PO43-(aq) 3Ca2(aq) 6Cl-(aq) ?
- 6K(aq) 6Cl-(aq) Ca3(PO4)2(s)
- Net Ionic Equation
- 2PO43-(aq) 3Ca2(aq) ? Ca3(PO4)2(s)
21
Mass - Mole Relationships of a Compound
For an Element
For a Compound
Mass (g) of Element
Mass (g) of compound
Moles of Element
Amount (mol) of compound
Amount (mol) of compound
Molecules (or formula units of compound)
Atoms of Element
22
Calculating the Number of Moles and Atoms in a
Given Mass of Element
Problem Tungsten (W) is the element used as the
filament in light bulbs, and
has the highest melting point of any element
3680oC. How many moles of
tungsten, and atoms of the
element are contained in a 35.0 mg sample of the
metal? Plan Convert mass into moles by dividing
the mass by the atomic weight of the
metal, then calculate the number of atoms by
multiplying by Avogadros
number! Solution Converting from mass of W to
moles Moles of W 35.0
mg W x 0.00019032
mol
1.90 x
10 - 4 mol NO. of W atoms 1.90 x 10 - 4 mol
W x
1.15 x 1020 atoms of Tungsten
1 mol W 183.9 g W
6.022 x 1023 atoms 1 mole of W
23
- Calcite is a mineral composed of calcium
carbonate, CaCO3. A sample of calcite composed of
pure calcium carbonate weighs 23.6 g. How many
moles of calcium carbonate is this?
First, find the molar mass of HNO3 1
Ca 1(40.08) 40.08 1 C 1(12.01) 12.01 3
O 3(16.00) 48.00
2 decimal places 100.09 g/mol
100.09
24
Next, find the number of moles in 23.6 g
25
- The daily requirement of chromium in the human
diet is 1.0 10-6 g. How many atoms of chromium
does this represent?
26
First, find the molar mass of Cr 1 Cr 1(51.996)
51.996
Now, convert 1.0 x 10-6 grams to moles
1.157781368 x 1016 atoms
1.2 x 1016 atoms (2 significant figures)
27
Chemical Formulas
Empirical Formula - Shows the relative number of
atoms of each element in the compound.
It is the simplest formula, and is
derived from masses of the elements. Molecular
Formula - Shows the actual number of atoms
of each element in the molecule of the
compound. Structural Formula - Shows the actual
number of atoms, and the bonds between
them that is, the arrangement of
atoms in the molecule.
28
- Percentage Composition
- The mass percentage of each element in the
compound - The composition is determined by experiment,
often by combustion. When a compound is burned,
its component elements form oxidesfor example,
CO2 and H2O. The CO2 and H2O are captured and
weighed to determine the amount of C and H in the
original compound.
29
- Lead(II) chromate, PbCrO4, is used as a paint
pigment (chrome yellow). What is the percentage
composition of lead(II) chromate?
First, find the molar mass of PbCrO4 1
Pb 1(207.2) 207.2 1 Cr 1(51.996) 51.996 4
O 4(16.00) 64.00
(1 decimal place) 323.2 g/mol
323.196
30
Now, convert each to percent composition
Check 64.11 16.09 19.80 100.00
31
Flow Chart of Mass Percentage Calculation
Moles of X in one mole of Compound
M (g / mol) of X
Mass (g) of X in one mole of compound
Divide by mass (g) of one mole
of compound
Mass fraction of X
Multiply by 100
Mass of X
32
Calculating Mass Percentage and Masses of
Elements in a Sample of a Compound - I
Problem Sucrose (C12H22O11) is common table
sugar. ( a) What is the mass percent of each
element in sucrose? ( b) How many grams of
carbon are in 24.35 g of sucrose?
(a) Determining the mass percent of each
element mass of C 12 x 12.01
g C/mol 144.12 g C/mol
mass of H 22 x 1.008 g H/mol
22.176 g H/mol mass of O 11 x
16.00 g O/mol 176.00 g O/mol
342.296 g/mol Finding
the mass fraction of C in Sucrose C
Total mass
of C 144.12 g C
mass of 1 mole of sucrose
342.30 g Cpd
Mass Fraction of C
0.421046 To find mass of C 0.421046 x
100 42.105
33
Calculating Mass Percents and Masses of Elements
in a Sample of Compound - II
(a) continued
Mass of H
x 100 x 100
6.479 H Mass of O
x 100
x 100
51.417 O (b) Determining the
mass of carbon Mass (g) of C mass of
sucrose X( mass fraction of C in sucrose) Mass
(g) of C 24.35 g sucrose X
10.25 g C
mol H x M of H 22 x
1.008 g H mass of 1 mol sucrose
342.30 g
mol O x M of O 11 x
16.00 g O mass of 1 mol sucrose
342.30 g
0.421046 g C 1 g sucrose
34
Empirical and Molecular Formulas
Empirical Formula - The simplest formula for a
compound that agrees
with the elemental analysis! The
smallest set of whole numbers of
atoms. Molecular Formula - The formula of the
compound as it exists,
it may be a multiple of the Empirical
formula.
35
Steps to Determine Empirical Formulas
Mass (g) of Element
M (g/mol )
Moles of Element
use no. of moles as subscripts
Preliminary Formula
change to integer subscripts
Empirical Formula
36
- Empirical Formula (Simplest Formula)
- The formula of a substance written with the
smallest integer subscripts - For example
- The empirical formula for N2O4 is NO2.
- The empirical formula for H2O2 is HO
37
Some Examples of Compounds with the Same
Elemental Ratios
Empirical Formula
Molecular Formula
CH2(unsaturated Hydrocarbons) C2H4 ,
C3H6 , C4H8 OH or HO
H2O2 S
S8 P
P4
Cl
Cl2 CH2O
(carbohydrates)
C6H12O6
38
- Determining the Empirical Formula
- Beginning with percent composition
- Assume exactly 100 g so percentages convert
directly to grams. - Convert grams to moles for each element.
- Manipulate the resulting mole ratios to obtain
whole numbers.
39
- Manipulating the ratios
- Divide each mole amount by the smallest mole
amount. - If the result is not a whole number
- Multiply each mole amount by a factor.
- For example
- If the decimal portion is 0.5, multiply by 2.
- If the decimal portion is 0.33 or 0.67, multiply
by 3. - If the decimal portion is 0.25 or 0.75, multiply
by 4.
40
- Benzene is composed of 92.3 carbon and 7.7
hydrogen. What is the empirical formula of
benzene?
Empirical formula CH
41
- Molecular Formula
- A formula for a molecule in which the subscripts
are whole-number multiples of the subscripts in
the empirical formula
42
- To determine the molecular formula
- Compute the empirical formula weight.
- Find the ration of the molecular weight to the
empirical formula weight. - Multiply each subscript of the empirical formula
by n.
43
Combustion Train for the Determination of the
Chemical Composition of Organic Compounds.
m 2
m 2
CnHm (n ) O2 n CO2(g) H2O(g)
Fig. 3.4
44
Determine composition by combustion
- Benzene is a liquid compound composed of carbon
and hydrogen it is used in the preparation of
polystyrene plastic. A sample of benzene weighing
342 mg is burned in oxygen and forms 1158 mg of
carbon dioxide. What is the percentage
composition of benzene?
45
Strategy 1. Use the mass of CO2 to find the mass
of carbon from the benzene. 2. Use the mass of
benzene and the mass of carbon to find the mass
of hydrogen. 3. Use these two masses to find the
percent composition.
46
First, find the mass of C in 1156 mg of CO2
315.5 mg C
47
Next, find the mass of H in the benzene sample
342 mg benzene -315.5 mg C 26.5 mg H (the
decimal is not significant)
- Now, we can find the percentage composition
48
Determining a Chemical Formula from
Combustion Analysis - I
Problem Erthrose (M 120 g/mol) is an
important chemical compound as
a starting material in chemical synthesis, and
contains Carbon Hydrogen, and
Oxygen. Combustion analysis of
a 700.0 mg sample yielded 1.027 g CO2 and
0.4194 g H2O. Plan We find the masses
of Hydrogen and Carbon using the mass
fractions of H in H2O, and C in CO2. The mass of
Carbon and Hydrogen are subtracted from
the sample mass to get the mass of
Oxygen. We then calculate moles, and construct
the empirical formula, and from the
given molar mass we can calculate the
molecular formula.
49
Determining a Chemical Formula from Combustion
Analysis - II
Calculating the mass fractions of the elements
Mass fraction of C in CO2
0.2729 g C
/ 1 g CO2 Mass fraction of H in H2O
0.1119 g H / 1 g H2O Calculating masses of C
and H Mass of Element mass of compound x
mass fraction of element
mol C x M of C mass of 1 mol CO2
1 mol C x 12.01 g C/ 1 mol C 44.01 g
CO2
mol H x M of H mass of 1 mol H2O
2 mol H x 1.008 g H / 1 mol H 18.02
g H2O
50
Determining a Chemical Formula from
Combustion Analysis - III
0.2729 g C 1 g CO2
Mass (g) of C 1.027 g CO2 x
0.2803 g C Mass (g) of H 0.4194 g H2O x
0.04693 g H Calculating
the mass of O Mass (g) of O Sample mass -(
mass of C mass of H )
0.700 g - 0.2803 g C - 0.04693 g H 0.37277 g
O Calculating moles of each element C
0.2803 g C / 12.01 g C/ mol C 0.02334 mol C
H 0.04693 g H / 1.008 g H / mol H 0.04656 mol
H O 0.37277 g O / 16.00 g O / mol O
0.02330 mol O C0.02334H0.04656O0.02330 CH2O
formula weight 30 g / formula 120 g /mol / 30 g
/ formula 4 formula units / cpd C4H8O4
0.1119 g H 1 g H2O
51
Some Compounds with Empirical Formula CH2O
(Composition by Mass 40.0 C, 6.71 H, 53.3O)
Molecular M Formula
(g/mol) Name Use or Function
CH2O 30.03 Formaldehyde
Disinfectant Biological
preservative C2H4O2 60.05
Acetic acid Acetate polymers vinegar
( 5 solution) C3H6O3
90.08 Lactic acid Causes milk
to sour forms
in muscle
during exercise C4H8O4 120.10
Erythrose Forms during sugar
metabolism C5H10O5
150.13 Ribose Component of
many nucleic
acids and
vitamin B2 C6H12O6 180.16
Glucose Major nutrient for energy
in cells
52
Ascorbic Acid ( Vitamin C ) - I Contains C , H ,
and O
- Upon combustion in excess oxygen, a 6.49 mg
sample yielded 9.74 mg CO2 and 2.64 mg H2O - Calculate its Empirical formula!
- C 9.74 x10-3g CO2 x(12.01 g C/44.01 g CO2)
- 2.65 x 10-3 g C
- H 2.64 x10-3g H2O x (2.016 g H2/18.02 gH2O)
- 2.92 x 10-4 g H
- Mass Oxygen 6.49 mg - 2.65 mg - 0.30 mg
- 3.54 mg O
53
Vitamin C Combustion - II
- C 2.65 x 10-3 g C / ( 12.01 g C / mol C )
- 2.21 x 10-4 mol C
- H 0.295 x 10-3 g H / ( 1.008 g H / mol H )
- 2.92 x 10-4 mol H
- O 3.54 x 10-3 g O / ( 16.00 g O / mol O )
- 2.21 x 10-4 mol O
- Divide each by 2.21 x 10-4
- C 1.00 Multiply each by 3 3.00 3.0
- H 1.32
3.96 4.0 - O 1.00
3.00 3.0
C3H4O3
54
H
H
H
C6H8O6
55
Molecular Formula
Molecules
Atoms
Avogadros Number
6.022 x 1023
Moles
Moles
56
Chemical Equations
Qualitative Information
Reactants
Products
States of Matter (s) solid (l)
liquid (g) gaseous (aq) aqueous
2 H2 (g) O2 (g) 2 H2O (g)
57
Chemical Equation Calculation - I
Atoms (Molecules)
Avogadros Number
6.02 x 1023
Molecules
Reactants
Products
58
Chemical Equation Calculation - II
Mass
Atoms (Molecules)
Molecular Weight
Avogadros Number
g/mol
6.02 x 1023
Molecules
Reactants
Products
Moles
59
- Stoichiometry
- The calculation of the quantities of reactants
and products involved in a chemical reaction - Interpreting a Chemical Equation
- The coefficients of the balanced chemical
equation may be interpreted in terms of either
(1) numbers of molecules (or ions or formula
units) or (2) numbers of moles, depending on your
needs.
60
Information Contained in a Balanced Equation
Viewed in Reactants
Products terms of 2 C2H6 (g)
7 O2 (g) 4 CO2 (g) 6 H2O(g) Energy
Molecules 2 molecules of C2H6 7 molecules of
O2
4 molecules of CO2 6 molecules of
H2O Amount (mol) 2 mol C2H6 7 mol O2
4 mol CO2 6 mol H2O Mass (amu) 60.14 amu
C2H6 224.00 amu O2
176.04 amu CO2
108.10 amu H2O Mass (g) 60.14 g C2H6
224.00 g O2 176.04 g CO2 108.10 g H2O Total
Mass (g) 284.14g
284.14g
61
- To find the amount of B (one reactant or product)
given the amount of A (another reactant or
product)
- 1. Convert grams of A to moles of A
- ? Using the molar mass of A
- 2. Convert moles of A to moles of B
- ? Using the coefficients of the balanced chemical
equation - 3. Convert moles of B to grams of B
- ? Using the molar mass of B
62
- Propane, C3H8, is normally a gas, but it is sold
as a fuel compressed as a liquid in steel
cylinders. The gas burns according to the
following equation - C3H8(g) 5O2(g) ? 3CO2(g) 4H2O(g)
- How many grams of CO2 are produced when 20.0 g of
propane is burned?
63
Molar masses C3H8 3(12.01) 8(1.008) 44.094
g CO2 1(12.01) 2(16.00) 44.01 g
59.9 g CO2 (3 significant figures)
64
- Propane, C3H8, is normally a gas, but it is sold
as a fuel compressed as a liquid in steel
cylinders. The gas burns according to the
following equation - C3H8(g) 5O2(g) ? 3CO2(g) 4H2O(g)
- How many grams of O2 are required to burn 20.0 g
of propane?
65
- Molar masses
- O2 2(16.00) 32.00 g
- C3H8 3(12.01) 8(1.008) 44.094 g
72.6 g O2 (3 significant figures)
66
- Limiting Reactant
- The reactant that is entirely consumed when a
reaction goes to completion - Once one reactant has been completely consumed,
the reaction stops. - Any problem giving the starting amount for more
than one reactant is a limiting reactant problem.
67
- All amounts produced and reacted are determined
by the limiting reactant. - How can we determine the limiting reactant?
- Use each given amount to calculate the amount of
product produced. - The limiting reactant will produce the lesser or
least amount of product.
68
- Magnesium metal is used to prepare zirconium
metal, which is used to make the container for
nuclear fuel (the nuclear fuel rods) - ZrCl4(g) 2Mg(s) ? 2MgCl2(s) Zr(s)
- How many moles of zirconium metal can be produced
from a reaction mixture containing 0.20 mol ZrCl4
and 0.50 mol Mg?
69
ZrCl4 is the limiting reactant. 0.20 mol Zr will
be produced.
70
- Urea, CH4N2O, is used as a nitrogen fertilizer.
It is manufactured from ammonia and carbon
dioxide at high pressure and high temperature - 2NH3 CO2(g) ? CH4N2O H2O
- In a laboratory experiment, 10.0 g NH3 and 10.0 g
CO2 were added to a reaction vessel. What is the
maximum quantity (in grams) of urea that can be
obtained? How many grams of the excess reactant
are left at the end of the reactions?
71
Molar masses NH3 1(14.01) 3(1.008) 17.02
g CO2 1(12.01) 2(16.00) 44.01
g CH4N2O 1(12.01) 4(1.008) 2(14.01)
1(16.00) 60.06 g
CO2 is the limiting reactant. 13.6 g CH4N2O will
be produced.
72
To find the excess NH3, we find how much NH3
reacted
Now subtract the amount reacted from the starting
amount
10.0 at start -7.73 reacted 2.27 g remains
2.3 g NH3 is left unreacted. (1 decimal place)
73
- Theoretical Yield
- The maximum amount of product that can be
obtained by a reaction from given amounts of
reactants. This is a calculated amount.
74
- Actual Yield
- The amount of product that is actually obtained.
This is a measured amount. - Percentage Yield
75
- 2NH3 CO2(g) ? CH4N2O H2O
- When 10.0 g NH3 and 10.0 g CO2 are added to a
reaction vessel, the limiting reactant is CO2.
The theoretical yield is 13.6 of urea. When this
reaction was carried out, 9.3 g of urea was
obtained. What is the percent yield?
Theoretical yield 13.6 g Actual yield 9.3 g
68 yield (2 significant figures)
76
Other Resources
- Visit the student website at college.hmco.com/pic/
ebbing9e
77
- The chemical name of table sugar is sucrose,
C12H22O11. How many grams of carbon are in 68.1 g
of sucrose.
First, find the molar mass of C12H22O11 12
C 12(12.01) 144.12 11 O 11(16.00) 176.00 22
H 22(1.008) 22.176
(2 decimal places) 342.30 g/mol
342.296
78
Now, find the mass of carbon in 61.8 g sucrose
79
- Sodium pyrophosphate is used in detergent
preparations. It is composed of 34.5 Na, 23.3
P, and 42.1 O. What is its empirical formula?
Empirical formula Na4P2O7
80
- Hexamethylene is one of the materials used to
produce a type of nylon. It is composed of 62.1
C, 13.8 H, and 24.1 N. Its molecular weight is
116 amu. What is its molecular formula?
Empirical formula C3H8N
81
- Sodium pyrophosphate is used in detergent
preparations. It is composed of 34.5 Na, 23.3
P, and 42.1 O. What is its empirical formula?
Empirical formula Na4P2O7
82
- Hexamethylene is one of the materials used to
produce a type of nylon. It is composed of 62.1
C, 13.8 H, and 24.1 N. Its molecular weight is
116 amu. What is its molecular formula?
Empirical formula C3H8N
83
- The empirical formula is C3H8N.
- Find the empirical formula weight
- 3(12.01) 8(1.008) 1(14.01) 58.104 amu
- Molecular formula C6H16N2
84
- Propane, C3H8, is normally a gas, but it is sold
as a fuel compressed as a liquid in steel
cylinders. The gas burns according to the
following equation - C3H8(g) 5O2(g) ? 3CO2(g) 4H2O(g)
- How many grams of CO2 are produced when 20.0 g of
propane is burned?
85
Molar masses C3H8 3(12.01) 8(1.008) 44.094
g CO2 1(12.01) 2(16.00) 44.01 g
59.9 g CO2 (3 significant figures)
86
- Propane, C3H8, is normally a gas, but it is sold
as a fuel compressed as a liquid in steel
cylinders. The gas burns according to the
following equation - C3H8(g) 5O2(g) ? 3CO2(g) 4H2O(g)
- How many grams of O2 are required to burn 20.0 g
of propane?
87
- Molar masses
- O2 2(16.00) 32.00 g
- C3H8 3(12.01) 8(1.008) 44.094 g
72.6 g O2 (3 significant figures)
88
Converting a Concentrated Solution to a Dilute
Solution