Recovery and Purification of Bio-Products - PowerPoint PPT Presentation
1 / 26
Title:
Recovery and Purification of Bio-Products
Description:
Recovery and Purification of Bio-Products Strategies to recovery and purify bio-products Fermenter Solid-liquid separation Cells Cell products Supernatant – PowerPoint PPT presentation
Number of Views:656
Avg rating:3.0/5.0
Title: Recovery and Purification of Bio-Products
1
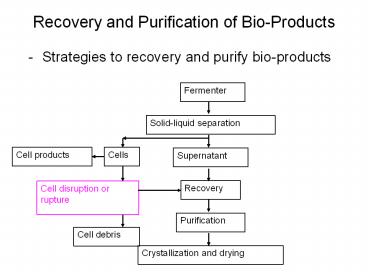
Recovery and Purification of Bio-Products
- Strategies to recovery and purify bio-products
Fermenter
Solid-liquid separation
Cells
Cell products
Supernatant
Recovery
Cell disruption or rupture
Purification
Cell debris
Crystallization and drying
2
Cell Disruption
- Disruption the cell envelope is physically
broken, releasing all intracellular components
into the surrounding medium - Methods Mechanical and non mechanical
- Mechanical
- - (sonicators)
- bacteria, virus and spores
- suspensions at lab-scale
- Electronic generator?ultrasonic waves
- ?mechanical oscillation
- by a titanium probe immersed
- in a cell disruption.
3
Cell Disruption
- Mechanical
- continuous operation,
- Algae, bacteria and fungi
- Large scale, up to 2000kg/h
- liquid and solid
- Principle of operation
- A grinding chamber filled with about 80 beads.
- A shaft with designed discs or impellers is
within the chamber. - The shift rotates at high speeds, high shearing
and impact forces from the beads break the cell
wall.
4
Cell Disruption
- Mechanical
- Ball Mill solid
- Frozen cell paste, cells attached to or within a
solid matrix. - Large scale
5
Cell Disruption
- Mechanical
- suspension, large
scale - To pump a slurry (up to 1500 bar) through a
restricted orifice valve. - The cells disrupt as they are extruded through
the valve to atmosphere pressure by - - high liquid shear in the orifice
- - sudden pressure drop upon discharge
- i.e. French press,
- Gaulin-Manton,
- Rannie high-pressure
- homogenizer
High pressure
orifice
6
Cell Disruption
- Nonmechanical
- - use chemicals to
solubilise the components in the cell walls to
release the product. - Chemical requirements
- - products are insensitive to the used
chemicals. - - the chemicals must be easily separable.
- Types of chemicals
- - surfactants (solubilising lipids) sodium
sulfonate, sodium dodecylsulfate. - - Alkali sodium hydroxide, harsh
- - Organic solvents penetrating the lipids and
swelling the cells. e.g. toluene. - e.g. Bacteria were treated with acetone followed
by sodium dodecyl sulfate extraction of cellular
proteins.
7
Cell Disruption
- Nonmechanical
- - to lyse cell walls to
release the product. - gentle, but high cost
- i.e. lysozyme (carbohydrase) to lyse the cell
walls of bacteria. - - .
- Osmosis is the transport of water molecules from
high- to a low-concentration region when these
two phases are separated by a selective membrane.
- Water is easier to pass the membrane than other
components. - When cells are dumped into pure water, cells can
swell and burst due to the osmotic flow of water
into the cells.
8
Cell Disruption
- Challenge Damage to the product
- Heat denaturation
- Oxidation of the product
- Unhindered release of all intracellular products
9
Recovery and Purification of Bio-Products
- Strategies to recovery and purify bio-products
Fermenter
Solid-liquid separation
Cells
Cell products
Supernatant
Recovery
Cell disruption or rupture
Purification
Cell debris
Crystallization and drying
10
Separation of Soluble Products
- Liquid-liquid extraction
- Difference of in two
liquids. - Applicable separate inhibitory fermentation
products such as ethanol and acetone-butanol from
fermentation broth. - antibiotics (i.e. solvent amylacetate)
- Requirements of liquid extractants
- nontoxic, selective, inexpensive, immiscible
with fermentation broth and - high distribution coefficient KDYL/XH
- YL and XH are concentrations of the solute in
light and heavy phases, respectively. - The light phase is the organic solvent and the
heavy phase is the fermentation broth. e.x.
Penicillin is extracted from a fermentation broth
using isoamylacetate. KD50.
Light, YL
Heavy, XH
11
Separation of Soluble Products
- Liquid-liquid extraction
- When fermentation broth contains more than one
component, then the selectivity coefficient (ß)
is important. - ßil KD,,i/KD,j
- KD,,I and KD,j are distribution coefficients of
component i and j. - The higher the value of ßil is, the easier the
separation of i from j. - pH effect, multi-stage extraction
12
Separation of Soluble Products
Reduce the
product solubility in the fermentation broth by
adding chemicals. Applicable separate proteins
or antibiotics from fermentation broth.
13
Separation of Soluble Products
- Precipitation
- Methods
- - by adding inorganic
salts such as ammonium sulfate, or sodium sulfate
to increase high ionic strength (factors pH,
temperature) - e.g. The solubility of hemoglobin is reduced
with increased amount of ammonium sulfate. - - added salts interact more stronger with water
so that the proteins precipitate. - - inexpensive
- - precipitation
- Precipitate a protein at its isoelectric point.
E.g. The IE of cytochrome cM (without histidine
tag) is 5.6 (Cho, et.al., 2000, Eur. J. Biochem.
267, 10681074).
14
Separation of Soluble Products
- Adsorption
- Adsorb soluble product from fermentation broth
onto solids. - Approaches physical adsorption, ion exchange
- Adsorption capacity mass of solute adsorbed per
unit mass of adsorbent - Affected by properties of adsorbents
- functional groups and their numbers, surface
properties - by properties of solution solutes, pH,
ionic strength and temperature - Difference of Affinity of product in the solid
and liquid phase. - Applicable soluble products from dilute
fermentation
15
Separation of Soluble Products
CHALLENGE!
SCREENING ADSORBENTS THE MOST PROMISING
TYPES - high capacity - reusable
16
Saturated uptake
Adsorbent 1
Adsorbent 2
Cs1
affinity
Cs2
C1
Adsorption Isotherms
17
Separation of Soluble Products
- Membrane separation
- Microfiltration 0.1 - 10 µm, bacterial and yeast
cells. - Ultrafiltration macromolecules (2000 ltMWlt
500,000) - Dialysis removal of low-MW solutes organic
acids (100ltMWlt500) and inorganic ions
(10ltMWlt100). - Reverse osmosis a pressure is applied onto a
salt-containing phase, which drives water from a
low to a high concentration region. MW lt 300. - The common features of the above methods
- .
- .
18
Separation of Soluble Products
Chromatography To separate the solutes based on
the different rate of movement of the solutes in
the column with adsorbent materials. Principles
Chromatographic processes involve a stationary
phase and a mobile phase. Stationary phase can
be adsorbent, ion-exchange resin, porous solid,
or gel usually packed in a cylindrical
column. Mobil phase is the solution containing
solutes to be separated and the eluant that
carriers the solution through the stationary
phase. Applicable for protein, organics
separation.
19
Separation of Soluble Products
Chromatography Method A solution containing
several solutes is injected at one end of the
column followed by the eluant carrying the
solution through the column. Each solutes in the
original solution moves at a rate proportional to
its relative affinity for the stationary phase
and comes out at the end of the column as a
separated band.
(M. Shuler, Bioprocess. Eng. 2005)
20
Separation of Soluble Products
Chromatography
- Mechanism
- Similar to adsorption interaction of
solute-adsorbent - Different to adsorption
- - Chromatography is based on different rate of
movement of the solute in the column - - Adsorption is based on the separation of one
solute from other constituents by being captured
on the adsorbent.
21
Separation of Soluble Products
Electrophoresis To separate charged solutes
based on their specific migration rates in an
electrical field. Positive charged solutes are
attracted to anode and negative charged solutes
to cathode. Factors electric field strength,
electric charge of the solutes, viscosity of
liquid and the particles size. Applicable for
protein separation.
22
Proteins Electrophoresis
http//fig.cox.miami.edu/cmallery/150/protein/SDS
.electrophoresis.jpg
23
Recovery and Purification of Bio-Products
- Strategies to recovery and purify bio-products
Fermenter
Solid-liquid separation
Cells
Cell products
Supernatant
Recovery
Cell disruption or rupture
Purification
Cell debris
Crystallization and drying
24
Recovery and Purification of Bio-Products
- Crystallization last step in producing highly
purified products such as antibiotics. - Supersaturated solution, low temperature,
- Crystals are separated by filters.
- Drying
- To remove solvent from purified wet product such
as crystal or dissolved solute. - Vaccum-tray dryers pharmaceutical products
- Freezing drying by sublimation (from solid ice
to vapor), antibiotics, enzyme, bacteria - Spray dryer heat-sensitive materials
25
Summary of separation and purification
- Liquid-Solid Separation
- - Filtration rotary vaccum drum filter, micro-
and ultra- filtration - - Centrifugation
- Cell disruption
- - Mechanical ultrasonication, milling,
homogenization - - Nonmechanical chemicals, enzyme and osmotic
shock
26
Summary of separation and purification
- Separation of soluble products
- - Liquid-liquid extraction
- - Precipitation
- - Adsorption
- - Membrane separation ultrafiltration,
dialysis, reverse osmosis - - Chromatography
- - Electrophoresis
- Crystallization and drying