NOW - PowerPoint PPT Presentation
1 / 46
Title: NOW
1
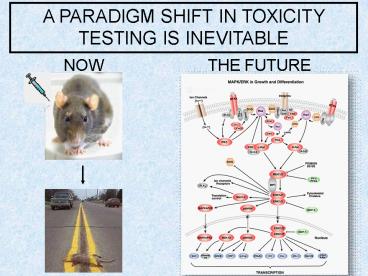
A PARADIGM SHIFT IN TOXICITY TESTING IS INEVITABLE
NOW
THE FUTURE
2
Risk Assessment Paradigm The Red Book
Approach (1983)
- Hazard identification animal studies
- Dose-response assessment animal studies
- Exposure assessment field studies
- Risk characterization hazard x exposure
- Risk Management exposure standard depends on
context, risk-benefit analysis
3
The Current Approach
- High doses in animals
- Large number of animals
- Low throughput
- Expensive
- Time consuming
- Pathology endpoints
- Dose response extrapolations over a wide range
- Application of uncertainty factors
4
The Future Approach
- Multiple doses in vitro
- Defined number of toxicity pathways
- High throughput
- Expensive to develop, cheap to do
- Fast
- Mechanistic endpoints
- In vitro-to-in vivo extrapolations of dose
response - Based on human biology
5
Blood Lead Levels in the U.S. Population
19761999 NHANES II, III, 99
lead paint Ban 1976
can solder phase-out Begins 1978
unleaded gasoline Introduced 1979
lead copper Rule 1991
can solder ends 1992
leaded gas ends 1996
6
Lanphear BP et. al., (2005). Low-Level
Environmental Lead Exposure and Children's
Intellectual Function An International Pooled
Analysis.Environ Health Perspect 113 894-899.
7
Needleman, HL. (1990). What Can the Study of Lead
Teach Us About Other Toxicants? Environ Health
Perspect 86183-189.
Landrigan, PJ et al. (2005). Early Environmental
Origins of Neurodegenerative Disease in Later
Life. Environ Health Perspect 1131230-1233.
8
(No Transcript)
9
How good is the current system?
- This is a difficult question to answer!
- For pharmaceuticals, some insight
- Olson, H et al. Concordance of Toxicity of
Pharmaceuticals in Humans and Animals, Regul
Toxicol Pharmacol 32, 56-67, 2000 - ?12 companies provided coded data to ILSI to
examine how well preclinical animal studies
predict actual human toxicities (150 compounds) - ?Overall true positive human toxicity concordance
of 71 (non-rodents alone 63, rodents alone 43) - ?Concordance varied a lot among different tissues
- ?Differences in metabolism dont explain
non-concordance
10
Released June 12, 2007 www.nas.edu
Toxicity Testing in the 21st CenturyA Vision and
A Strategy
Committee on Toxicity Testing and Assessment of
Environmental Agents Board on Environmental
Studies and Toxicology Institute for Laboratory
Animal Research Division on Earth and Life
Studies National Research Council
11
Committee Membership
Daniel Krewski (Chair), University of Ottawa,
Ottawa, ON Daniel Acosta, Jr., University of
Cincinnati, Cincinnati, OH Melvin Andersen, CIIT
Centers for Health Research, Research Triangle
Park, NC Henry Anderson, Wisconsin Division of
Public Health, Madison, WI John Bailar III,
University of Chicago, Chicago, IL Kim
Boekelheide, Brown University, Providence,
RI Robert Brent, Thomas Jefferson University,
Wilmington, DE Gail Charnley, HealthRisk
Strategies, Washington, DC Vivian Cheung,
University of Pennsylvania, Philadelphia,
PA Sidney Green, Howard University, Washington,
DC Karl Kelsey, Harvard University, Boston,
MA Nancy Kerkvliet, Oregon State University,
Corvallis, OR Abby Li, Exponent, Inc., San
Francisco, CA Lawrence McCray, Massachusetts
Institute of Technology, Cambridge MA Otto Meyer,
Danish Institute for Food and Veterinary
Research, Søborg, Denmark D. Reid Patterson, Reid
Patterson Consulting, Inc., Grayslake, IL William
Pennie, Pfizer, Inc., Groton, CT Robert Scala,
Exxon Biomedical Sciences (Ret.), Tucson, AZ Gina
Solomon, Natural Resources Defense Council, San
Francisco, CA Martin Stephens, The Humane Society
of the United States, Washington, DC James Yager,
Jr., Johns Hopkins University, Baltimore,
MD Lauren Zeise, California Environmental
Protection Agency, Oakland, CA
12
Design Criteria Objectives of Toxicity Testing
13
Options for Future Toxicity Testing Strategies
14
Options for Future Toxicity Testing Strategies
15
Options for Future Toxicity Testing Strategies
16
Options for Future Toxicity Testing Strategies
17
Options for Future Toxicity Testing Strategies
18
The Vision
19
(No Transcript)
20
(No Transcript)
21
Proposed new direction based on Toxicity
Pathways
TOXICITY PATHWAYS Cellular response pathways
that, when sufficiently perturbed, are predictive
of an adverse health effect
22
Toxicity Pathways The Task
- Identify the toxicity pathways
- Understand their interactions
- Determine dose-dependent changes in function
- Build confidence in the distinction between
ADAPTIVE (homeostatic and reversible) and ADVERSE
(unstable, progressive, and permanent) effects
23
Toxicity Pathways How to Start
- Take a dozen or so human cell types in vitro
- Genetically manipulate these cells
- Knockouts
- Transgenics
- siRNAs
- Challenge the cells with classes of toxicants
- Measure pathway alterations, applying omics
technology
24
Heres an idea miRNA KOs
- There are 600-800 miRNA in humans
- There is probably a lot of redundancy
- Each miRNA epigenetically modulates the activity
of multiple pathways - Take 30-50 canonical miRNA KO human cell lines
and test toxicant dose-responses - Advantages include reasonable pathway modulation
and broad pathway coverage
25
26
Describe the System Quantitatively - Convert
Signaling Diagrams Into Computational Models
- Characterize the transitions of molecular species
with time - State variables molecular species (Gene A, Gene
B, etc) - Generic activation
- r dB/dt kactA kdeactB
- Generic repression
- r dC/dt Ract kdeactB
- Specific bimolecular reactions
- E S ? ES ? E P
- r dE/dt -k1ES (k2k3)ES
27
(No Transcript)
28
Understanding Dose Response
NFkB Modeling with MAP3K1 Cross-Talk
29
Computational Systems Biology Model for the
Circuitry and the Output
Circuitry models developed for all key assays to
support dose response assessment, from bottom up
30
Higher Dose
Higher yet
31
(No Transcript)
32
(No Transcript)
33
Toxicity Pathway Results and Quantitative Risk
Assessments
34
PROMISES
- Human relevance
- Dose relevance
- Chemical coverage
- Mixtures effects on toxicity pathways
- Mechanistic focus mode of action based
- Cost effective
- Fast
- The 3 Rs replacement, reduction, refinement
35
CONUNDRUMS
- Screening tool or stand-alone test system?
- Validation to what? Animals at high doses?
- Human cell lines have a lot of abnormal biology
- Mixture effects that are indirect
- Metabolism
- Epigenetics, and other unknown mechanisms
- Cell-cell and organ interactions
- Distinguishing adaptive from adverse
responses - Toxicogenomics overpromised underperformed?
- Use of an unfamiliar surrogate (rats look more
like people than cells look like people) - Is this another war on cancer?
36
10s/year
100s/year
10,000s/day
100,000s/day
1-3/year
High Throughput
Molecular mechanism
Immediate Human Relevance
37
Collins, Gray, Bucher Transforming Environmental
Health Protection Science 319906-07 (2008)
38
Toxicity Testing and Risk Assessment
Dose Response Assessment
Chemical Characterization
Mode of Action
Population Based Studies
Compounds
Dose Response Analysis for Perturbations of
Toxicity Pathways
Affected Pathway
Assess Biological Perturbation
Calibrating in vitro and human Dosimetry
Exposure Guideline
Measures of dose in vitro
Metabolite(s)
Human Exposure Data
Hazard Identification
Risk Characterization
39
Regulatory Context
- Shift in focus away from apical outcomes in
experimental animals towards important
perturbations of toxicity pathways - Development of risk assessment practices based on
pathway perturbations
- Re-interpretation or possible re-writing of
regulatory statues under which risk assessments
are conducted
40
(No Transcript)
41
Discussion Points
- The vision is too grandiose, too futuristic, and
will take too long to implement. - What evidence is there that toxicity pathways can
be defined from in vitro systems and will be of
practical, decision making utility? - Is it really possible to extrapolate to humans
and define relevant effect and no effect levels
from in vitro dose response modeling based on
toxicity pathway perturbations? - The vision is often justified as being cost
effective and efficient, but maybe replacing our
current approach, that we understand and feel
comfortable with, is just cutting corners and
circumventing due diligence. - Many safety issues are due to highly complex
phenomena, like development and neurotoxicity, or
low incidence outcomes, like cardiotoxicity. How
can simple in vitro models predict these type of
issues?
42
(No Transcript)
43
Toxicity Testing in Practice
- Ethylhexyl methoxycinnamate (EHMC)
- A very common UV filter in sunscreen
- Reviewed by the NTP BSC as a proposed research
project. Drs. Kerkvliet and Boekelheide are the
reviewers
44
The Concern
- Widespread use
- Lifelong exposure
- Potential for endocrine disruption
- Potential for increased absorption in children
- Lack of information on the effects of in utero
exposure
45
The Limited Information Generates Questions
- Industry says it has a study that clears EHMC of
concerns as an endocrine disruptor, but the data
are not public - Reasonably strong evidence that absorption
through the skin is most often very limited (1) - Sunlight causes a large amount of EHMC
isomerization - Metabolism generates 2-ethylhexanol and
2-ethylhexanoic acid, known developmental
toxicants - Nanoparticles now widely used in sunscreens have
unknown effects on transdermal transport - Young age and some common skin conditions
(eczema) may enhance transdermal absorption
46
The NTP Proposal
- Evaluate toxicokinetics and ADME, comparing
dermal and oral routes of exposure - Conduct a robust ORAL multigenerational study
- (high dose at MTD, low dose orders of magnitude
above anticipated exposure levels)
The BSC Recommendation
- High priority for toxicokinetics and ADME,
comparing dermal and oral routes - Low-to-moderate priority for a multigenerational
study

























![READ⚡[PDF]✔ Let Us Now Praise Famous Men PowerPoint PPT Presentation](https://s3.amazonaws.com/images.powershow.com/10048407.th0.jpg?_=202406050911)
![⚡Read✔[PDF] Now I Know: The Soviets Invaded Wisconsin?!: ...And 99 More Interesting Facts, PowerPoint PPT Presentation](https://s3.amazonaws.com/images.powershow.com/10071901.th0.jpg?_=20240704011)



