Chapter 11: Phase Transformations - PowerPoint PPT Presentation
1 / 50
Title:
Chapter 11: Phase Transformations
Description:
Nucleation is the process whereby nuclei (seeds) act as templates for crystal growth. ... Alloying elements used to modify the critical cooling rate for martensite are ... – PowerPoint PPT presentation
Number of Views:3474
Avg rating:5.0/5.0
Title: Chapter 11: Phase Transformations
1
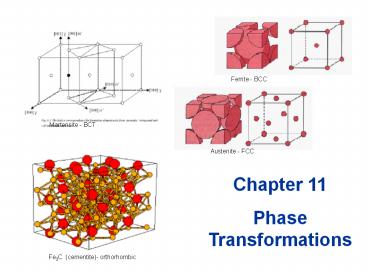
Ferrite - BCC
Martensite - BCT
Austenite - FCC
Chapter 11 Phase Transformations
Fe3C (cementite)- orthorhombic
2
Phase Transformations
- Transformation rate
- Kinetics of Phase Transformation
- Nucleation homogeneous, heterogeneous
- Free Energy, Growth
- Isothermal Transformations (TTT diagrams)
- Pearlite, Martensite, Spheroidite, Bainite
- Continuous Cooling
- Mechanical Behavior
- Precipitation Hardening
3
Phase Transformations
- Phase transformations change in the number or
character of phases. - Simple diffusion-dependent
- No change in of phases
- No change in composition
- Example solidification of a pure metal,
allotropic transformation, recrystallization,
grain growth - More complicated diffusion-dependent
- Change in of phases
- Change in composition
- Example eutectoid reaction
- Diffusionless
- Example metastable phase - martensite
4
Phase Transformations
- Most phase transformations begin with the
formation of numerous small particles of the new
phase that increase in size until the
transformation is complete. - Nucleation is the process whereby nuclei (seeds)
act as templates for crystal growth. - Homogeneous nucleation - nuclei form uniformly
throughout the parent phase requires
considerable supercooling (typically 80-300C). - Heterogeneous nucleation - form at structural
inhomogeneities (container surfaces, impurities,
grain boundaries, dislocations) in liquid phase
much easier since stable nucleating surface is
already present requires slight supercooling
(0.1-10ºC).
5
Supercooling
- During the cooling of a liquid, solidification
(nucleation) will begin only after the
temperature has been lowered below the
equilibrium solidification (or melting)
temperature Tm. This phenomenon is termed
supercooling (or undercooling. - The driving force to nucleate increases as ?T
increases - Small supercooling ? slow nucleation rate - few
nuclei - large crystals - Large supercooling ? rapid nucleation rate - many
nuclei - small crystals
6
c11f01
Nucleation of a spherical solid particle in a
liquid
- The change in free energy DG (a function of the
internal energy and enthalpy of the system) must
be negative for a transformation to occur. - Assume that nuclei of the solid phase form in the
interior of the liquid as atoms cluster
together-similar to the packing in the solid
phase. - Also, each nucleus is spherical and has a radius
r. - Free energy changes as a result of a
transformation 1) the difference between the
solid and liquid phases (volume free energy,
DGV) and 2) the solid-liquid phase boundary
(surface free energy, DGS). - Transforming one phase into another takes time.
Liquid
DG DGS DGV
7
Homogeneous Nucleation Energy Effects
r critical nucleus for r lt r nuclei shrink
for r gtr nuclei grow (to reduce energy)
8
Solidification
Note ?Hf and ? are weakly dependent on ?T
9
Transformations Undercooling
g
Þ
a
Fe3C
Eutectoid transformation (Fe-Fe3C system)
For transformation to occur, must cool to below
727C
0.76 wt C
6.7 wt C
0.022 wt C
10
FRACTION OF TRANSFORMATION
Fraction transformed depends on time.
Transformation rate depends on T.
r often small equil not possible
2
11
Generation of Isothermal Transformation Diagrams
Consider
The Fe-Fe3C system, for Co 0.76 wt C A
transformation temperature of 675C.
100
T 675C
transformed
50
0
2
4
time (s)
1
10
10
12
Eutectoid Transformation Rate DT
Coarse pearlite ? formed at higher temperatures
relatively soft Fine pearlite ? formed at
lower temperatures relatively hard
13
Nucleation and Growth
Reaction rate is a result of nucleation and
growth of crystals.
Examples
5
14
c11f13
Isothermal Transformation Diagrams
- 2 solid curves are plotted
- one represents the time required at each
temperature for the start of the transformation - the other is for transformation completion.
- The dashed curve corresponds to 50 completion.
- The austenite to pearlite transformation will
occur only if the alloy is supercooled to below
the eutectoid temperature (727C). - Time for process to complete depends on the
temperature.
15
c11f14
Isothermal Transformation Diagram
Eutectoid iron-carbon alloy composition, Co
0.76 wt C Begin at T gt 727C Rapidly cool
to 625C and hold isothermally.
Austenite-to-Pearlite
16
Transformations Involving Noneutectoid
Compositions
Consider C0 1.13 wt C
Hypereutectoid composition proeutectoid
cementite
17
c11f37
Possible Transformations
18
c11f15
Coarse pearlite (high diffusion rate) and (b)
fine pearlite
19
Bainite Non-Equil Transformation Products
- elongated Fe3C particles in a-ferrite matrix
- diffusion controlled
- a lathes (strips) with long rods of Fe3C
Martensite
Cementite
Ferrite
20
Bainite Microstructure
- Bainite consists of acicular (needle-like)
ferrite with very small cementite particles
dispersed throughout. - The carbon content is typically greater than
0.1. - Bainite transforms to iron and cementite with
sufficient time and temperature (considered
semi-stable below 150C).
21
Spheroidite Nonequilibrium Transformation
- Fe3C particles within an a-ferrite matrix
- diffusion dependent
- heat bainite or pearlite at temperature just
below eutectoid for long times - driving force reduction of a-ferrite/Fe3C
interfacial area
10
22
c11f20
Pearlitic Steel partially transformed to
Spheroidite
23
Martensite Formation
Martensite needles
Austenite
- single phase
- body centered tetragonal (BCT) crystal structure
- BCT if C0 gt 0.15 wt C
- Diffusionless transformation
- BCT ? few slip planes ? hard, brittle
- transformation depends only on T of rapid
cooling
24
An micrograph of austenite that was polished flat
and then allowed to transform into martensite.
The different colors indicate the displacements
caused when martensite forms.
25
Isothermal Transformation Diagram
- Iron-carbon alloy with eutectoid composition.
- A Austenite
- P Pearlite
- B Bainite
- M Martensite
26
c11f24
Effect of Adding Other Elements
4340 Steel
- Other elements (Cr, Ni, Mo, Si and W) may cause
significant changes in the positions and shapes
of the TTT curves - Change transition temperature
- Shift the nose of the austenite-to-pearlite
transformation to longer times - Shift the pearlite and bainite noses to longer
times (decrease critical cooling rate) - Form a separate bainite nose
nose
plain carbon steel
- Plain carbon steel primary alloying element is
carbon.
27
c11f23
- Example 11.2
- Iron-carbon alloy with eutectoid composition.
- Specify the nature of the final microstructure (
bainite, martensite, pearlite etc) for the alloy
that is subjected to the following
timetemperature treatments - Alloy begins at 760C and has been held long
enough to achieve a complete and homogeneous
austenitic structure. - Treatment (a)
- Rapidly cool to 350 C
- Hold for 104 seconds
- Quench to room temperature
Bainite, 100
28
c11f23
- Example 11.2
- Iron-carbon alloy with eutectoid composition.
- Specify the nature of the final microstructure (
bainite, martensite, pearlite etc) for the alloy
that is subjected to the following
timetemperature treatments - Alloy begins at 760C and has been held long
enough to achieve a complete and homogeneous
austenitic structure. - Treatment (b)
- Rapidly cool to 250 C
- Hold for 100 seconds
- Quench to room temperature
Austenite, 100
Martensite, 100
29
c11f23
- Example 11.2
- Iron-carbon alloy with eutectoid composition.
- Specify the nature of the final microstructure (
bainite, martensite, pearlite etc) for the alloy
that is subjected to the following
timetemperature treatments - Alloy begins at 760C and has been held long
enough to achieve a complete and homogeneous
austenitic structure. - Treatment (c)
- Rapidly cool to 650C
- Hold for 20 seconds
- Rapidly cool to 400C
- Hold for 103 seconds
- Quench to room temperature
Austenite, 100
Almost 50 Pearlite, 50 Austenite
Bainite, 50
Final 50 Bainite, 50 Pearlite
30
c11f26
Continuous Cooling Transformation Diagrams
- Isothermal heat treatments are not the most
practical due to rapidly cooling and constant
maintenance at an elevated temperature. - Most heat treatments for steels involve the
continuous cooling of a specimen to room
temperature. - TTT diagram (dotted curve) is modified for a CCT
diagram (solid curve). - For continuous cooling, the time required for a
reaction to begin and end is delayed. - The isothermal curves are shifted to longer times
and lower temperatures.
31
c11f27
- Moderately rapid and slow cooling curves are
superimposed on a continuous cooling
transformation diagram of a eutectoid iron-carbon
alloy. - The transformation starts after a time period
corresponding to the intersection of the cooling
curve with the beginning reaction curve and ends
upon crossing the completion transformation
curve. - Normally bainite does not form when an alloy is
continuously cooled to room temperature
austenite transforms to pearlite before bainite
has become possible. - The austenite-pearlite region (A---B) terminates
just below the nose. Continued cooling (below
Mstart) of austenite will form martensite.
32
c11f28
- For continuous cooling of a steel alloy there
exists a critical quenching rate that represents
the minimum rate of quenching that will produce a
totally martensitic structure. - This curve will just miss the nose where pearlite
transformation begins
33
c11f29
- Continuous cooling diagram for a 4340 steel alloy
and several cooling curves superimposed. - This demonstrates the dependence of the final
microstructure on the transformations that occur
during cooling. - Alloying elements used to modify the critical
cooling rate for martensite are chromium, nickel,
molybdenum, manganese, silicon and tungsten.
34
Mechanical Properties
- Hardness
- Brinell, Rockwell
- Yield Strength
- Tensile Strength
- Ductility
- Elongation
- Effect of Carbon Content
35
c11f30
Mechanical Properties Influence of Carbon
Content
Pearlite (med)
ferrite (soft)
C0 lt 0.76 wt C
Hypoeutectoid
36
c11f31
Mechanical Properties Fe-C System
37
c11f34
Tempered Martensite
- Tempered martensite is less brittle than
martensite tempered at 594 C. - Tempering reduces internal stresses caused by
quenching. - The small particles are cementite the matrix is
a-ferrite. US Steel Corp.
4340 steel
38
c11f33
Hardness as a function of carbon concentration
for steels
39
c11f36
Rockwell C and Brinell Hardness
Hardness versus tempering time for a
water-quenched eutectoid plain carbon steel
(1080) room temperature.
40
c11tf02
41
Precipitation Hardening
- The strength and hardness of some metal alloys
may be improved by the formation of extremely
small, uniformly dispersed particles
(precipitates) of a second phase within the
original phase matrix. - Other alloys that can be precipitation hardened
or age hardened - Copper-beryllium (Cu-Be)
- Copper-tin (Cu-Sn)
- Magnesium-aluminum (Mg-Al)
- Aluminum-copper (Al-Cu)
- High-strength aluminum alloys
42
c11f40
Phase Diagram for Precipitation Hardened Alloy
- Criteria
- Maximum solubility of 1 component in the other
(M) - Solubility limit that rapidly decreases with
decrease in temperature (M?N). - Process
- Solution Heat Treatment first heat treatment
where all solute atoms are dissolved to form a
single-phase solid solution. - Heat to T0 and dissolve B phase.
- Rapidly quench to T1
- Nonequilibrium state (a phase solid solution
supersaturated with B atoms alloy is soft,
weak-no ppts).
43
c11f43
Precipitation Heat Treatment the 2nd stage
- The supersaturated a solid solution is usually
heated to an intermediate temperature T2 within
the ab region (diffusion rates increase). - The b precipitates (PPT) begin to form as finely
dispersed particles. This process is referred to
as aging. - After aging at T2, the alloy is cooled to room
temperature. - Strength and hardness of the alloy depend on the
ppt temperature (T2) and the aging time at this
temperature.
44
Precipitation Hardening
Particles impede dislocation motion. Ex
Al-Cu system Procedure
-- Pt A solution heat treat (get a solid
solution)
-- Pt B quench to room temp. (retain a solid
solution)
-- Pt C reheat to nucleate small q particles
within a phase.
At room temperature the stable state of an
aluminum-copper alloy is an aluminum-rich solid
solution (a) and an intermetallic phase with a
tetragonal crystal structure having nominal
composition CuAl2 (?).
45
c11f43
Precipitation Heat Treatment the 2nd stage
- PPT behavior is represented in the diagram
- With increasing time, the hardness increases,
reaching a maximum (peak), then decreasing in
strength. - The reduction in strength and hardness after long
periods is overaging (continued particle growth).
Small solute-enriched regions in a solid solution
where the lattice is identical or somewhat
perturbed from that of the solid solution are
called Guinier-Preston zones.
46
PRECIPITATION STRENGTHENING
Hard precipitates are difficult to shear.
Ex Ceramics in metals (SiC in Iron or Aluminum).
Result
24
47
c11f44
- Several stages in the formation of the
equilibrium PPT (q) phase. - supersaturated a solid solution
- transition (q) PPT phase
- equilibrium q phase within the a matrix phase.
48
Influence of Precipitation Heat Treatment on
Tensile Strength (TS), EL
2014 Al Alloy
TS peak with precipitation time. Increasing
T accelerates process.
49
c11f45
Effects of Temperature
- Characteristics of a 2014 aluminum alloy (0.9 wt
Si, 4.4 wt Cu, 0.8 wt Mn, 0.5 wt Mg) at 4
different aging temperatures.
50
Aluminum rivets
- Alloys that experience significant precipitation
hardening at room temp and after short periods
must be quenched to and stored under refrigerated
conditions. - Several aluminum alloys that are used for rivets
exhibit this behavior. They are driven while
still soft, then allowed to age harden at the
normal room temperature.