Net Ionic Equations - PowerPoint PPT Presentation
Title:
Net Ionic Equations
Description:
H (aq) OH-(aq) H2O(l) Sodium ion and chloride ion are 'spectator ions' ... In pure water, a few molecules ionize to form H3O and OH. H2O H2O OH H3O ... – PowerPoint PPT presentation
Number of Views:172
Avg rating:3.0/5.0
Title: Net Ionic Equations
1
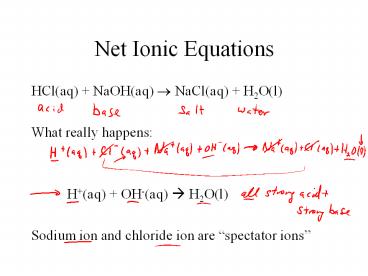
Net Ionic Equations
- HCl(aq) NaOH(aq) ? NaCl(aq) H2O(l)
- What really happens
- H(aq) OH-(aq) ? H2O(l)
- Sodium ion and chloride ion are spectator ions
2
Reactions involving weak bases
- HCl(aq) NH3(aq) ? NH4(aq) Cl-(aq)
- Net-Ionic Equation
3
What are the products CH3CO2H(aq) NaOH(aq)
?
- 1. CH3CO2H2(aq) NaO(aq)
- 2. CH3CO2-(aq) H2O(l) Na(aq)
- 3. CH4(g) CO2(g) H2O(l)
Net-Ionic Equation
4
What are the products HCN(aq) NH3(aq)
?
- 1. NH4(aq) CN-(aq)
- 2. H2CN(aq) NH2-(aq)
- 3. C2N2(s) 3 H2(g)
- Net-ionic equation
5
Solution Concentration Molarity
- Molarity moles solute per liter of solution
- 0.30 mol NH3 dissolved in 0.500 LConcentration
- Written like NH3
0.60 M - If you dissolve 0.32 mol NH3 to make 750 mL of
solution, what is NH3?
6
pH Quantitative Measure of Acidity
- Acidity is related to concentration of H (or
H3O) - pH -logH H 10-pH
7
pH Scale
- In pure water, a few molecules ionize to form
H3O and OHH2O H2O ? OH H3O - In acidic and basic solutions, these
concentrations are not equalacidic H3O gt
OHbasic OH gt H3Oneutral H3O
OH
8
pH Scale
- Measure how much H3O is in a solution using pH
- pH lt 7.0 acidic
- pH gt 7.0 basic
- pH 7.0 neutral
- Measure of H3O and OH concentration (moles per
liter) in a solution - As acidity increases, pH ?
9
pH Scale
- The pH scale is logarithmic100 102 log(102)
210 101 log(101) 11 100 log(100)
00.1 101 log(101) 10.01 102 log(102)
2 - pH log H
10
pH Scale
- pH log H3O
- What is the pH if H 1 x 105? Acidic or
basic? - What is the pH if H 1 x 109? Acidic or
basic? - What is the pH if H 0.000057 M?
11
pH of Strong Acid Solutions
For monoprotic strong acids H acid What
is the pH of a 2.4 x 10-2 M solution of HNO3?
12
Finding H3O from pH
- H 10-pH
- What is H if pH 8.9?
13
What is the possible range of pH values?