Thermodynamics - PowerPoint PPT Presentation
1 / 42
Title:
Thermodynamics
Description:
Heat Capacity of Heating an Ideal Monatomic Gas ... Cv and Cp of Monatomic Gas. Cv and Cp of H2O at 373K. 2 mol of monatomic ideal gas. Calculate q, w, U and H ... – PowerPoint PPT presentation
Number of Views:60
Avg rating:3.0/5.0
Title: Thermodynamics
1
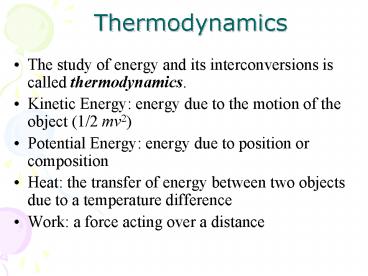
Thermodynamics
- The study of energy and its interconversions is
called thermodynamics. - Kinetic Energy energy due to the motion of the
object (1/2 mv2) - Potential Energy energy due to position or
composition - Heat the transfer of energy between two objects
due to a temperature difference - Work a force acting over a distance
2
Figure 9.1 (a) Initial position of balls
3
Figure 9.1 (b) Final position of balls
4
Energy of Matters
5
State Function
- A property of the system depends only on its
present state. A state function does not depend
in any way on the systems past. - Energy is a state function, but work and heat are
not state function
6
Combustion of methane
7
Nitrogen/oxygen
8
First Law of Thermodynamics
- The energy of the universe is constant
In closed system ?E ?Uqw q the heat added to
the system during the process w the work done on
the system during the process qgt0 heat flows into
the system from the surroundings qlt0 an outflow
of heat from the system to the surroundings wgt0
work is done on the system by the
surroundings wlt0 the system does work on the
surroundings
9
P-V Work
10
P-V Work
11
Enthalpy
- The heat qp absorbed in a constant-pressure
- process equals the systems enthalpy change.
12
- For a chemical reaction
- ?H?Hproducts-?Hreactants
- If ?Hreactantsgt?Hproducts (endothermic)
- If ?Hreactantslt?Hproducts (exothermic)
- Consider a constant-volume process
- dw-PdV0
- ?Uqwqv
- ?Uqv
13
Thermodynamics of Ideal Gases
14
(No Transcript)
15
Heat Capacity of Heating an Ideal Monatomic Gas
- Under constant volume, the energy flowing into
the gas is used to increase the translational
energy of the gas molecules.
16
Heat Capacity of Ideal Monatomic Gases
17
Heat Capacity of Diatomic Gases
18
Heat Capacity of Polyatomic Gases
19
Heat Capacity of Heating a Polyatomic Gas
- Polyatomic gases have observed values for Cv that
are significantly greater than 3/2 R. - This larger value for Cv results because
polyatomic molecules absorb energy to excite
rotational and vibrational motions in addition to
translational motions.
20
Cv and Cp of molecules
21
Cv and Cp of Monatomic Gas
22
Cv and Cp of H2O at 373K
23
(No Transcript)
24
2 mol of monatomic ideal gas Calculate q, w, ?U
and ?H for both pathway
25
- TA122K, TC366K, TB183K, TD61K
- Cv3/2R, Cp5/2R
- Path 1(A?C)
- w1-P?V-2atm(30-10)L101.3J/Latm-4.05103J
- q1qpnCp?T25/2(R)(366-122)1.01104J?H1
- ?U1nCv?T 23/2(R)(366-122)6.08103J
- Path 2(C?B)
- q2qvnCv?T23/2(R)(183-366)-4.56103J?U2
- ?H2nCp?T 25/2(R)(183-366)-7.6103J
- ?V0 w2 -P?V 0
26
- Path 3(A?D)
- q3qvnCv?T23/2(R)(61-122)-1.52103J?U3
- ?H3nCp?T 25/2(R)(61-122)-2.53103J
- ?V0 w2 -P?V 0
- Path 4(D?B)
- w1-P?V-1atm(30-10)L101.3J/Latm-2.03103J
- q4qpnCp?T25/2(R)(183-61)5.08104J?H4
- ?U4nCv?T 23/2(R)(183-61)3.05103J
27
Summary
- Path 1
- qpath1q1q25.5 103J
- wpath1w1w2 -4.05103J
- ?Hpath1 ?H1 ?H2 2.55103J
- ?Upath1 ?U1 ?U2 1.52103J
- Path 2
- qpath2q3q43.56103J
- wpath2w3w4 -2.03103J
- ?Hpath2 ?H3 ?H4 2.55103J
- ?Upath2 ?U3 ?U4 1.52103J
28
(No Transcript)
29
Calorimetry
- Specific heat capacity with unit JK-1g-1
- Molar heat capacity with unit Jk-1mol-1
30
Coffee Cup Calorimeter
- A constant-pressure calorimetry is used in
determining the change in enthalpy equals the
heat. - ?HqpnCp?T
31
2SO2(g)O2(g)?2SO3(g) ?H-198 KJ 2 mol. 1
mol. 2 mol. Calculate ?H and ?U
32
- P is constant, ?Hqp-198 KJ (energy flow out of
system) - ?U qp w
- w-P?V and ?V?n(RT/P)
- T and P are constant, ?nnfinal-ninitial-1 mol.
- So w-P?V-P?n (RT/P)
- - ?nRT-(-1)(8.314)(298)2.48 KJ
- ?U qp w-198KJ2.48KJ-196KJ
33
Bomb Calorimeter
- ?V0
- ?Uqv0qv
- ?UqvnCv ? T
34
Hesss Law
- If a reaction is carried out in a series of
steps, ?H for the reaction will be equal to the
sum of the enthalpy changes for the individual
steps - The overall enthalpy change for the process is
independent of the number of steps or the
particular nature of the path by which the
reaction is carried out.
35
- Consider the combustion reaction of methane to
- form CO2 and liquid H2O
- CH4(g) 2O2(g)? CO2(g) 2H2O(l)
- ?H1 -890KJ/mol
- This reaction can be thought of as occurring in
- two steps
- CH4(g) 2O2(g)? CO2(g) 2H2O(g)
- ?H2 -802 kJ/mol
- 2H2O(g)?2H2O(l)
- ?H3 -88KJ/mol
- ?H1 ?H2 ?H3
36
(No Transcript)
37
Standard Enthalpies of Formation
- The change in enthalpy that accompanies the
formation of 1 mole of a compound from its
elements with all substances in their standard
states. - The superscript zero indicates that the
corresponding process has been carried out under
standard conditions.
38
(No Transcript)
39
(No Transcript)
40
(No Transcript)
41
Present Sources of Energy
- Petroleum and Natural Gas
- Coal
New Energy Sources
Coal Conversion Hydrogen as a fuel
42
(No Transcript)















![L 18 Thermodynamics [3] PowerPoint PPT Presentation](https://s3.amazonaws.com/images.powershow.com/6877662.th0.jpg?_=20150709116)














