Nuclear Chemistry - PowerPoint PPT Presentation
1 / 20
Title:
Nuclear Chemistry
Description:
fraction of a radioisotope remaining after n half-lives is ( )n. mfinal ... How long is 3 half-lives? Problem: Thorium-234 has a half-life of 25 days. ... – PowerPoint PPT presentation
Number of Views:66
Avg rating:3.0/5.0
Title: Nuclear Chemistry
1
(No Transcript)
2
(No Transcript)
3
Nuclear Reactions
- result in a change in the atoms nucleus.
- Example
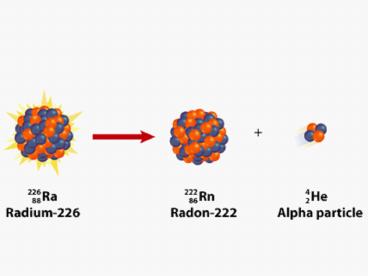
- Ra ? Rn a
226 88
222 86
42
4
226 88
42
222 86
Rn
- Ra ? a
5
238 92
42
234 90
- U ? a
Th
6
14 6
0-1
14 7
- C ? ß
N
7
9236
141 56
235 92
10
- U n? Ba Th ?
10
3 n
8
Problem
- Write the equation for I undergoing beta
decay.
131 53
9
Problems
223 88
42
- Ra ? ? a
- a N ? ? H
- Rb ? ? ?
11
42
14 7
8738
8737
10
Problems
21
- H ? ? He
- C ? ? ß
42
14 6
0-1
11
Write a balanced nuclear equation for the alpha
decay of the following radioisotope
234 92
U
12
230 90
4 2
U ? Th ?
234 92
13
(No Transcript)
14
Write the symbols for the followingneutron alp
haproton beta(electron) gammapositron
15
neutron proton electronpositronalphabetaga
mma
- n
- p
- e e
- ?? ??
10
11
00
0-1
01
42
0-1
00
16
Half-Life (t1/2)
- time required for half of the atoms in a sample
to decay.
17
Radioactive Decay Rates
The percent of a stontium-90 sample remaining
over a period of four half-lives.
18
Determining Decay
- fraction of a radioisotope remaining after n
half-lives is (½)n - mfinal minitial (½)n
19
Problem
- N-13 has a half-life of 10 minutes. How much of
2.00 g. N-13 will exist after 3 half-lives? - How long is 3 half-lives?
20
Problem
- Thorium-234 has a half-life of 25 days. How much
of the original will remain after 50 days?