Scientific Notation - PowerPoint PPT Presentation
1 / 10
Title:
Scientific Notation
Description:
Adjust decimal place of header if necessary so that it is between 1 and 10. ... based on the atomic clock. 1.7. Standards for SI Base Units ... – PowerPoint PPT presentation
Number of Views:88
Avg rating:3.0/5.0
Title: Scientific Notation
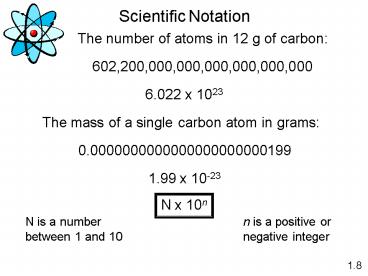
1
Scientific Notation
6.022 x 1023
1.99 x 10-23
N x 10n
N is a number between 1 and 10
n is a positive or negative integer
1.8
2
Scientific Notation
Multiplication/Division
(4.0 x 10-5) x (7.0 x 103) (4.0 x 7.0) x
(10-53) 28 x 10-2 2.8 x 10-1
- Multiply N1 and N2
- Add exponents n1 and n2
- Adjust decimal place of header if necessary so
that it is between 1 and 10. Change power of
exponent accordingly - Lower it by one for each place the decimal moves
right. - Raise it by one for each place the decimal moves
left.
8.5 x 104 5.0 x 109 (8.5 5.0) x 104-9 1.7
x 10-5
1.8
3
Scientific Notation
Addition or Subtraction
(8.7 x 104) (9.6 x 105) (0.87 x 105) (9.6 x
105) (0.87 9.6) x 105 10.5 x 105 1.05 x
106
- Convert smaller number to have the same power as
the larger number - Add or subtract N1 and N2
- Result has same power
- Adjust decimal place if header is not between 1
and 10
1.8
4
Standards for SI Base Units
Standards are defined by the General Conference
on Weights and Measures (GCPM)
- committee of 18 elected scientists
- head the International Committee of Weights and
Measures, who make decisions about the operation
of the Bureau of Weights and Measures.
- meets every 4-6 years to reevaluate standards
- located on an estate in Severs, France, near
Paris (where metric system was invented by the
French Academy of Scientists in 1795)
1.7
5
Standards for SI Base Units
meter (1790) 1/10,000,000th the distance
between the North Pole and the Equator as
measured through Paris, France.
meter (1870) length between two marks on a
Pt-Ir bar stored in a climate controlled vault in
Severs, France.
meter (modern) the distance a beam of light
travels through a vacuum in 1/299,792,458th of a
second.
- based on the laser
1.7
6
Standards for SI Base Units
second (ancient) 1/86,400th of a day, the time
it takes the sun to move to the same position in
the sky (60 second 1 minutes, etc.)
second (modern) the duration of 9,192,63,770
vibrations of a light wave emitted by a
cesium-133 atom as its electrons transition
between the two lowest energy states (the
hyperfine levels of the ground state)
- based on the atomic clock
1.7
7
Standards for SI Base Units
kilogram(1795-1889) the mass of 1000 cubic
centimeters of water at its highest density (4oC).
kilogram(1889-modern) the mass of the
International Prototype Kilogram (IPK)
- a chunk of metal stored in an environmentally
controlled vault in Severs, France.
1.7
8
1.7
9
1.7
10
Useful Conversion Factors
- 1 miles 1609 m 1.609 km
- 1 miles 5280 feet
- 1 lbs (of mass) 454 g
- 1 in 2.54 cm
- 1 ft 0.3048 m or 1 m 3.28 m