Molecular Diagnosis - PowerPoint PPT Presentation
1 / 71
Title:
Molecular Diagnosis
Description:
A locus on the human X chromosome contains such a stretch of nucleotides in ... Stasis hypoxia and ischemic infarction of liver, kidney, heart, bone, nervous system ... – PowerPoint PPT presentation
Number of Views:3118
Avg rating:3.0/5.0
Title: Molecular Diagnosis
1
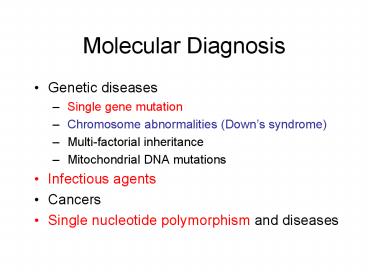
Molecular Diagnosis
- Genetic diseases
- Single gene mutation
- Chromosome abnormalities (Downs syndrome)
- Multi-factorial inheritance
- Mitochondrial DNA mutations
- Infectious agents
- Cancers
- Single nucleotide polymorphism and diseases
2
Molecular diagnosisGenetic testing
- Reference Book
- Human Molecular Genetics
- 3rd Edition
- ISBN 0-8153-4184-9
3
Introduction
- The choice of materials to test
- DNA
- RNA
- Protein
- Three possible questions
- Gene testing has to be targeted.
- Does the patient have any mutation in this
particular gene that may cause disease. - Does the patient have a 3-base deletion of the
codon for F508 in his CFTR gene?
4
RNA has advantages and disadvantages
- Advantages
- Without introns
- RT-PCR can detect aberrant splicing
- Disadvantages
- Less convenient to obtain and work with
- Handle with extreme care and process rapidly to
avoid degradation - Gene of interest may not be expressed in readily
accessible tissues - Many mutation results in mRNA instability
5
Genetic diseasesSingle gene mutation
- Autosomal dominant disorders
- e.g. Huntingtons disease (CAG)n
- Autosomal recessive disorders
- e.g. b- thalassemia
- Sex-linage inheritance disorders
- X-linked dominant
- ?????
- ?????? ????
- ??????
- ??????????heterozygotes ????
- ?fragile X
- X-linked recessive
- ????????
- ????
- ????
- ?DMD????
6
(No Transcript)
7
Molecular basis of hereditary diseases
- Huntingtons disease
- (CAG)n
- Myotonic dystrophy
- (CTG)n or (CCTG)n
- Fragile X chromosomes
- (CGG)n
- Cystic fibrosis
- (single nucleotide change of CFTR)
- Duchenne muscular dystrophy
- (65 deletion, 30 non-sense mutation)
- Sickle cell anemia
- (b chain 6th amino acid E? V substitution)
- b-thalassemia
- Diminished (b or b)or absent (b0 ) of b-globin
8
Laboratory diagnosis of trinucleotide repeat
diseases
- Huntington disease (CAG)n
- polyglutamine repeat
- A fragment is amplified by PCR, PAGE, silver
stain - lane 1,2,6,10 are normal, others are affected
people - Myotonic dystrophy (large expansion)
- Southern blot, bands of 9,10 kB are normal
- Fragile X (CGG)n
- Southern blot, DNA is digested with EclXI EcoRI
- The DNA of the inactivated female is methylated
- EclXI is sensitive to methylation (do not
cut) - Hybridize to Ox1.9 probe
9
- The protein made by the Huntington's gene is
called huntingtin - Huntingtin indirectly leads to nerve cell damage
and toxicity is through the formation of protein
aggregates and neuronal inclusions.
10
Huntingtons disease
11
Isolation of Huntingtons gene
12
FISH
13
(No Transcript)
14
(No Transcript)
15
Detection of trinucleotide repeat diseases I
(Huntington disease)
16
Myotonic dystrophy (??????)
- Type I, Type II, and congenital type
17
Myotonic dystrophy
DM1 and CMyD are caused by an abnormal
trinucleotide (CTG) repeat expansion in the DM1
locus on chromosome 19q13.3. DM2 is caused by
an abnormal tetranucleotide (CCTG) repeat
expansion in the DM2 locus on chromosome 3q21.
18
Detection of trinucleotide repeat diseases II
(Myotonic dystrophy)
19
Fragile X chromosome
- Characterizied by satellite regions visible at
the ends of metaphase chromosomes. These are due
to a long series of CGG triplet repeats. (due to
backward slippage of daughter strand) - prominent and elongated ears and long face.
- Most of the affected males have mental
retardation, and their testes are larger than
normal.
20
Fragile X chromosomes
Fragile X chromosomes are characterizied by
satellite regions visible at the ends of
metaphase chromosomes. Fragile X is associated
with Martin-Bell Syndrome, the most common form
of inherited predisposition to mental retardation
A locus on the human X chromosome contains such a
stretch of nucleotides in which the triplet CGG
is repeated 5-50X
21
Detection of trinucleotide repeat diseases III
(Fragile X)
F Fully expanded and methylated NM
Methylated X do not cut with EclXI P
Unmethylated premutation N X in normal male
and active normal female
22
Scanning a gene for mutation
- Methods based on sequencing
- Methods based on detecting mismatches or
hetero-duplexes - Methods based on single-strand conformation
analysis (SSCP) - Methods based on translation
- Methods for detecting deletion
- Methods for detecting DNA methylation
23
Cystic fibrosis
- The defect in a single gene results in the
production of abnormally viscous mucus secretions
causing recurrent chest infections, pancreatic
insufficiency, malabsorption of food and
intestinal obstruction in the newborn (meconium
ileus). - Almost all are single nucleotide mutation in CFTR
gene.
24
CF and DMD pose rather different sets of problem
for DNA diagnosis
25
Diagram of how cystic fibrosis is inherited
26
(No Transcript)
27
(No Transcript)
28
Methods based on sequencing
- Sequencing is becoming cheaper and easier (high
throughput) ? meta PCR of a large gene - Alternative methods are because they are cheaper
(e.g. SSCP), quicker (e.g.dHPLC), or give some
special information (PTT and quantitative PCR), - PTT is (protein truncation test)
- Exon average 145 bp, a sequencing run 500-800 bp.
? meta-PCR
29
Meta-PCR (exon-linking PCR)
30
Mutation detection by sequencing
31
(No Transcript)
32
Methods based on detecting hetero-duplexes
- Most mutations occurs in heterozygous form.
- Heteroduplex can be formed simply by heating the
heterozygous test PCR product to denature and
then to cooling slowly. - For homozygous or X-linked ? add some reference
wild type DNA - Heteroduplexes have abnormal motility on
nondenaturing gels.
33
Mutation scanning by dHPLC
34
Scanning the CTFR gene for mutationsby SSCP,
heteroduplex and DGGE (denaturing gradient gel
electrophoresis)
SSCP
DGGE
Heteroduplex variants
35
- Heteroduplex and SSCP analysis
- Upper panel (SSCP)
- Single stranded DNA run more slowly in the same
gel - Lane 1,2 wild type
- Lane 3-8 variants
- Lower panel (Heteroduplex)
- Heteroduplex in lane 3-8
- Denaturing gradient gel electrophoresis (DGGE)
- Exon PCR of CFTR in 9 urea-formaldehyde
denaturants - Usually splits into many sub-bands
- Subject A has variant in amplicon 6
- Subject B has variants in amplicon 17 and 24.
36
(No Transcript)
37
Methods based on detecting mismatches
- Chemical cleavage of mismatch (CCM)
- Cleavage by osmium tetraoxide (very toxic)
- Cleavage by KMnO4
- Enzymatic cleavage of mismatch (ECM)
- Endonulcease VII
- T4 resovase
- Only dHPLC is widely used in major diagnostic
laboratory
38
Mutation scanning by chemical (hydroxylamine)
cleavage of mismatches
39
Methods based on translation
- PTT is a specific test for frameshift, splice
site or nonsense mutations that create a
premature termination codon. - It is not useful in mutation like cystic fibrosis
where mutations are not truncated. - It reveal approximate location of any mutation
- Technical problems???
40
DMD mutation scanning using PTT(protein
truncation test)
41
Duchenne Muscular Dystrophy (???????? )
- The etiological cause of DMD is the genetic
mutation of the dystrophin gene that lead to the
absence or diminution of dystrophin protein in
muscle cells. - Dystrophin gene is the largest gene so far
identified, which spans approximately 2.5 million
base pairs on the X-chromosome
42
CF and DMD pose rather different sets of problem
for DNA diagnosis
43
Roles of Dystrophin in striatal muscle cell
- Dystrophin is an enormous rod-like protein (427
kDa) localized beneath the inner surface of
plasma membrane of mature striatal muscle cell
(myofibers), both skeletal and cardiac. - The N-terminal domain binds to the F-actin of
cytoskeletal structures, while the C-terminal
cysteine-rich domain along with the distal
C-terminus, anchors to the plasma membrane
through dystrophin-associated (DAP) glycoprotein
complexes. - Thus, dystrophin crosslinks and stabilizes the
cell membrane and the cytoskeletal structures. - In Duchenne muscular dystrophy muscle, the
absence of dystrophin also leads to the
diminution of the DAP complex including
dystroglycans and sarcoglycans.
44
Symptoms of Duchenne Muscular Dystrophy
- Enlarged calves and progressive muscle weakness
and failure are the hallmark signs. - Specific early clinical symptoms can include
- Delayed onset of walking
- Difficulty in performing a standing jump
- A waddled (????) walk
- Difficulty in getting up from the floor.
- Specific late clinical symptoms can include
- Difficulty in getting up from a chair
- The loss of ability to normally climb stairs
- A very wide gaited walk with balance problems
45
(No Transcript)
46
Methods for detecting deletion
- Homozygous or hemizygous deletion are simple to
detect (must R/O technical failure) - Heterozygous deletion of one or more whole exons
are not detectable when genomic DNA is amplified
exon by exon - (SSCP and heterduplex ? negative because of
whole exon deletion) - Two or more multiplex PCR reactions will reveal
98 of the deletions.
47
Multiplex screen for dystrophin deletions in males
48
Quantitative gene dosage to detect heterozygous
deletion in genomic DNA
- Real time PCR
- By cleavage of a sequence specific
oligonucleotide labeled with a dye and a quencher - MAPH (Multiplex Amplifiable Probe Hybridization)
- Genomic DNA is spotted onto a tiny membrane
- Hybridize to a mixture of 40 probes each
specific for an exon of a gene - after wash ? multiplex PCR
- exons can be distinguished by the length of the
PCR products
49
During PCR the labeled oligonucleotide hybridizes
to the target sequence and the 5'-dye is removed
by 5' 3'-exonuclease activity of Taq. The
fluorescence of the donor is no longer quenched
and can be measured.
50
Multiplex amplifiable probe hybridization (MAPH)
51
(No Transcript)
52
Detection of DNA methylation
- Excessive or deficient methylation of CpG is a
common pathogenic mechanism in cancer and
imprinted gene expression - Restriction enzyme HpaII cuts only unmethylated
CCGG whereas MspI cuts any CCGG - Bisulfite seqeuncing ? when single stranded DNA
is treated with sodium bisulfite, C ? U but 5-MeC
is not converted to U
53
Restriction enzyme that recognizes DNA methylation
54
(No Transcript)
55
Testing for a specified sequence change
- Is much simpler problem than scanning a gene for
presence of any mutation. - Diagnosis of dieases with limited allelic
heterogeneity (see table 18-4) - Diagnosis within a family
- SNP genotyping ? to test a DNA sample for a
pre-defined sequence variant.
56
(No Transcript)
57
(No Transcript)
58
(No Transcript)
59
Many simple methods are available for genotyping
a specified variant
- Allele specific oligonucleotide (ASO)
- Allele specific PCR amplification
- Amplification Refractory Mutation System (ARMS)
- Oligonucleotide ligation assay (OLA)
- Oligonucleotide array for mutation detection
- By hybridization to DNA array (e.g. Affimetrix
array) - Arrayed Primer Extension (APEX)
60
Sickle cell anemia
- B chain 6th amino acid E? V substitution results
in polymerization of deoxy form within the red
cells - The replacement of A by T at the 17th nucleotide
of the gene for the beta chain of hemoglobin
changes the codon GAG (E) to GTG (V). - The sickled-shape cells block microcirculation
- Stasis ? hypoxia and ischemic infarction of
liver, kidney, heart, bone, nervous system - Hemolytic anemia or even DIC
61
(No Transcript)
62
ASO
63
Amplification Refractory mutation system (ARMS)
64
(No Transcript)
65
Multiplex ARMS to detect cystic fibrosis
66
Introducing an artificial diagnostic restriction
site
67
Mutant has no ScaI site
68
Oligonucleotide ligation assay (OLA)
- Two oligonucleotides are constructed that
hybridize to adjacent sequences in the target,
with join sited at the position of the mutation. - DNA ligase will not join the two oligonucleotide
unless they are perfectly hybridized.
69
(No Transcript)
70
Oligonucleotide ligation assay (OLA) using OLA
to test 31 hot spots in CFTR
71
(No Transcript)