Ch' 8 Alkyl Halides - PowerPoint PPT Presentation
1 / 54
Title:
Ch' 8 Alkyl Halides
Description:
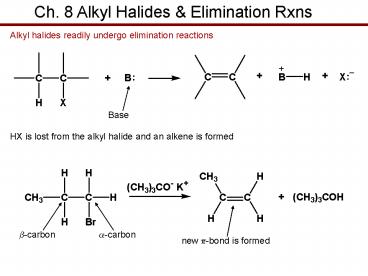
HX is lost from the alkyl halide and an alkene is formed. b-carbon. a-carbon. new p-bond is formed ... Reactions of Secondary Alkyl Halides ... – PowerPoint PPT presentation
Number of Views:351
Avg rating:3.0/5.0
Title: Ch' 8 Alkyl Halides
1
Ch. 8 Alkyl Halides Elimination Rxns
Alkyl halides readily undergo elimination
reactions
Base
HX is lost from the alkyl halide and an alkene is
formed
2
Common Bases
DBN DBU
amidines
3
Alkenes
The sp2 carbons have a planar geometry, bond
angles are 120o The C-C double bond cannot
rotate
4
Classify the following alkene.
A) monosubstituted B) disubstituted C)
trisubstituted D) tetrasubstituted
5
Classify the following alkene.
A) monosubstituted B) disubstituted C)
trisubstituted D) tetrasubstituted
6
Alkenes
What is the relationship between these two
structures?
7
Cis/Trans Isomerism in Alkenes
trans-2-butene cis-2-butene
Cis/Trans isomers are diastereomers Each double
bond C must be bonded to two different groups for
stereoisomers to be possible
8
Which of the following can have
another stereoisomer?
A B
C
D E
9
Stability of Alkenes
The stability of an alkene increases with
substitution of the double bond
Increasing number of R groups Increasing stability
10
Stability of Alkenes
Trans alkenes are more stable than cis alkenes
11
Which of the following alkenes is more stable?
A B
12
Which of the following alkenes is more stable?
A B
13
Mechanisms of Elimination Rxns
Two common mechanisms of elimination 1. E2
mechanism (bimolecular elimination) Similar
to SN2 Loss of leaving group and p-bond
formation occur at the same time
2. E1 mechanism (unimolecular elimination)
Two step mechanism (like SN1) Loss of
leaving group forms a carbocation p-bond is
formed in a second step
14
E2 Elimination
E2 Elimination (bimolecular elimination) Most
common elimination mechanism Rate depends upon
concentration of halide and base Rate
k R-X Base Example
15
E2 Elimination
The E2 reaction is concerted bond
breaking/forming happens in a single step
new p-bond
The base removes a hydrogen atom from the b-carbon
16
E2 Elimination
17
Rate of E2 Elimination
1. Base the rate of the reaction increases with
base strength
3. Solvent Polar aprotic solvents increase the
rate of E2 reactions Anions are not well
solvated
18
Rate of E2 Elimination
4. Alkyl halide 3o gt 2o gt 1o (opposite SN2
trend)
19
Which E2 reaction is more rapid?
A B
20
Which E2 reaction is more rapid?
A B
21
Zaitsevs Rule
For some elimination reactions, more than one
product is possible
22
Zaitsevs Rule
23
What is the major product of the following E2
reaction?
A B
C
D E
24
What is the major product of the following E2
reaction?
A B C
D E
25
Geometry of E2 Elimination
E2 elimination takes place from the anti
periplanar conformation This can influence
The stereochemistry of the alkene product The
location of the double bond (for compounds
with cyclohexane rings)
26
E2 Elimination in Cyclohexane Rings
For elimination to occur the H removed and the
leaving group must be trans diaxial (anti
periplanar)
axial b hydrogens
leaving group is axial
27
E2 Elimination in Cyclohexane Rings
The trans diaxial orientation can lead to
formation of the less substituted alkene
(contrary to Zaitsevs rule)
28
STEREOCHEMICAL CONSEQUENCES
Since the pi bond must form during the transition
state, the two bonds being broken must be
parallel (normally anti-). This produces
stereospecificity and causes substrates that can
not achieve this conformation to react slowly or
not at all.
STEREOSPECIFICITY
29
1) Now make the two hydrogen atoms different as
shown below and here is what you get.
2) Reverse the identity of the two hydrogen atoms
and the results of the experiment are reversed.
30
1) Here is what is happening.
31
You do the next one
32
What product would you get from the following?
Only one is formed in each case.
33
How about from the other diastereomer?
34
E1 Elimination
E1 Elimination (unimolecular elimination)
Two-step elimination mechanism (similar to SN1)
Generally occurs with SN1 and results in a
product mixture (less useful)
Rate depends upon concentration of halide
35
The E1 Elimination Mechanism
2. Weak bases favor E1 (strong bases favor E2)
3. E1 reactions follow Zaitsevs rule (the more
substituted alkene is favored)
36
What is the major elimination product?
A B
C
D E
37
Predicting Reaction Types
How can we predict whether substitution or
elimination occurs? How can we identify the
reaction mechanism?
1. Classify the alkyl halide as 3o, 2o, or 1o.
This is the most important factor. 2. Classify
the nucleophile/base as strong or weak.
Determine if a bases are bulky.
38
Reactions of Primary Alkyl Halides
Primary alkyl halides only react by SN2 and E2
pathways SN1 and E1 reactions would require
formation of an unstable primary carbocation SN2
is favored, even with basic nucleophiles, unless
a strong bulky base is used
39
Reactions of Secondary Alkyl Halides
Secondary alkyl halides react by all pathways
(SN1, SN2, E1, E2) depending on the
conditions Weak, nonbasic nucleophiles (H2O,
ROH) result in a mixture of SN1 and E1 products
40
Reactions of Secondary Alkyl Halides
Strong, nonbasic nucleophiles (X-, RS-, CN-, N3-)
favor SN2
41
Reactions of Secondary Alkyl Halides
Strong bases that are too sterically hindered to
be good nucleophiles give E2 elimination
42
Reactions of Tertiary Alkyl Halides
Tertiary halides can react by E2, E1, and
SN1 Strong bases (OH-, RO-, DBU) favor E2
43
Which reaction type is favored? A) SN1 B)
SN2 C) E1 D) E2 E) SN1 and E1 F) SN2 and
E2
44
Which reaction type is favored? A) SN1 B)
SN2 C) E1 D) E2 E) SN1 and E1 F) SN2 and
E2
45
Which reaction type is favored? A) SN1 B)
SN2 C) E1 D) E2 E) SN1 and E1 F) SN2 and
E2
46
Which reaction type is favored? A) SN1 B)
SN2 C) E1 D) E2 E) SN1 and E1 F) SN2 and
E2
47
Which reaction type is favored? A) SN1 B)
SN2 C) E1 D) E2 E) SN1 and E1 F) SN2 and
E2
48
Which reaction type is favored? A) SN1 B)
SN2 C) E1 D) E2 E) SN1 and E1 F) SN2 and
E2
49
Which reaction type is favored? A) SN1 B)
SN2 C) E1 D) E2 E) SN1 and E1 F) SN2 and
E2
50
Which reaction type is favored? A) SN1 B)
SN2 C) E1 D) E2 E) SN1 and E1 F) SN2 and
E2
51
What is the BEST way to prepare this alkene?
A B
C D
52
What is the BEST way to prepare this ether?
A B
C
D E
53
These structures are- A) Constitutional
isomers B) Enantiomers C) Unrelated D)
Diastereomers E) Identical
54
These structures are- A) Constitutional
isomers B) Enantiomers C) Unrelated D)
Diastereomers E) Identical