Keep It Simple - PowerPoint PPT Presentation
1 / 51
Title:
Keep It Simple
Description:
... get the chemical formula of an ionic compound if you know the two elements in it. ... Because those elements can form multiple oxidation states and you use ... – PowerPoint PPT presentation
Number of Views:144
Avg rating:3.0/5.0
Title: Keep It Simple
1
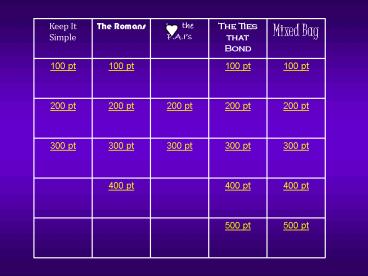
Keep It Simple
The Romans
I the P.A.Is
The Ties that Bond
Mixed Bag
100 pt
100 pt
100 pt
100 pt
200 pt
200 pt
200 pt
200 pt
200 pt
200 pt
300 pt
300 pt
300 pt
300 pt
300 pt
300 pt
400 pt
400 pt
400 pt
500 pt
500 pt
2
Describe how you get the chemical formula of an
ionic compound if you know the two elements in
it.
3
You take the absolute value of the charges and
cross them so that the charge on one atom becomes
the subscript of the other atom.
4
What is the chemical formula of the ionic
compound formed by aluminum and sulfur?
5
Al2S3
6
Name this compound MgBr2
7
Magnesium bromide
8
Using the balancing of charges method, explain
how you get the chemical formula for the ionic
compound formed by calcium and iodine.
9
Calcium forms an ion with a 2 charge and iodine
forms an ion with a -1 charge. You need two
iodine atoms to balance out the 2 charge from
calcium (the overall compound has to be neutral).
10
(No Transcript)
11
(No Transcript)
12
For which elements do you have to include a Roman
numeral in the name? How many exceptions are
there???
13
Transition metals, lead and tin. There are 10
exceptions Zn, Cd, Ag, Tc, Ta, V, Zr, Hf, Y and
Sc..
14
Why do you have to use Roman numerals in the name
of certain cations?
15
Because those elements can form multiple
oxidation states and you use the Roman numeral to
indicate which oxidation state is present in the
compound.
16
- Name these ionic compounds
- SnCl4
- Fe3N4
17
- Tin IV chloride
- Iron IV nitride
18
- Name these ionic compounds
- MnO
- FeN
19
- Manganese II oxide
- Iron III nitride
20
(No Transcript)
21
(No Transcript)
22
- How are the atoms in a polyatomic ion bonded?
- How do polyatomic ions bond to other atoms?
23
- By covalent bonds
- In an ionic manner
24
- Name these ionic compounds
- Mg(OH)2
- Al2(CO3)3
- NaC2H3O2
25
- Magnesium hydroxide
- Aluminum carbonate
- Sodium acetate
26
- Name these ionic compounds
- Cu(NO3)2
- Ti3(AsO4)2
- Fe2(SO4)3
27
- Copper II nitrate
- Titanium II arsenate
- Iron III sulfate
28
- Name these ionic compounds
- CuCO3
- HgHCO3
- FeAsO4
29
- Copper II carbonate
- Mercury I bicarbonate
- Iron III arsenate
30
Daily Double
31
- Give possible formulas for these polyatomic ions
- Hypophosphite
- Phosphite
- Perphosphate
PO2-3
PO3-3
PO5-3
32
Explain how two ions form an ionic bond.
33
The two ions are attracted to each other by the
electrostatic attraction between opposite
charges.
34
What do you call this structure
35
A crystal lattice structure
36
When is the bonding distance between ions in a
crystal lattice structure finalized?
37
When the attractive forces between the ions
balance out the repulsive forces between the
ions.
38
What is lattice energy and what is it used to
measure?
39
Lattice energy is the energy released when one
mole of an ionic crystal is formed from gaseous
ions. Its used to measure the strength of ionic
attraction in an ionic compound. Higher lattice
energy stronger compound.
40
Why is energy released when gaseous ions come
together to form a solid? Why is the sign for
this energy negative?
41
Energy is released because ions in the gas state
have more energy than ions in the solid state.
When the gas ions form a solid, the excess energy
is released. The sign is negative to indicate
energy is flowing out of the system.
42
What state are ionic compounds in at room
temperature? Can they conduct electricity in
that state?
43
Theyre solids, and no, they cant conduct
electricity as solids.
44
Why can the elements in the d-block (plus lead
and tin) have more than one oxidation state?
45
Because they have electrons in the d sublevel.
This allows them to be stable in many different
electron configurations, resulting in the
formation of multiple cations.
46
Which of these two ionic compounds would you
expect to have the higher boiling point LiF
(lattice energy1036 kJ/mol) OR KF (lattice
energy781 kJ/mol)?
47
LiF because it has the higher lattice energy
which means there is a stronger attraction
between molecules so more energy will be needed
to separate the solid ions into the gas state.
48
Diagram what happens when you dissolve calcium
nitride into a solution. Explain how this allows
the solution to conduct electricity.
49
When an ionic compound is dissolved into a
solution, it breaks up into its ions.
Ca3N2
Ca2 N-3 Ca2 N-3 Ca2 N-3
The presence of these charged particles in the
solution allows electricity to pass through it.
50
Why does MgCl2 have a higher lattice energy (2526
kJ/mol) than KCl (715 kJ/mol)?
51
Because magnesium has a higher charge than
potassium. A higher charge means a stronger
attraction which requires more energy to break
it.






![READ [PDF] Cash Drawer Tracker: A Logbook To Keep Track Your Total Cash On Hand And Coins PowerPoint PPT Presentation](https://s3.amazonaws.com/images.powershow.com/10086543.th0.jpg?_=20240726072)















![[PDF] READ Free Lord Keep Your Arm Around My Shoulder and Your Hand Over My PowerPoint PPT Presentation](https://s3.amazonaws.com/images.powershow.com/10050024.th0.jpg?_=20240607086)







