Characteristics of Photoluminescence: - PowerPoint PPT Presentation
1 / 12
Title:
Characteristics of Photoluminescence:
Description:
Phosphorescence involves change in electron spin and ... Types of Fluoresence/phosphorescence: ... More often, molecular fluorescence (phosphorescence) occurs ... – PowerPoint PPT presentation
Number of Views:6081
Avg rating:3.0/5.0
Title: Characteristics of Photoluminescence:
1
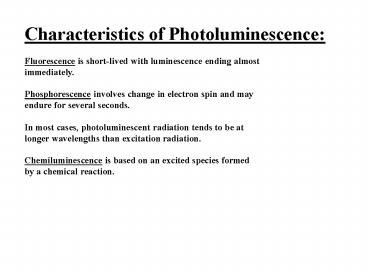
Characteristics of Photoluminescence Fluorescenc
e is short-lived with luminescence ending
almost immediately. Phosphorescence involves
change in electron spin and may endure for
several seconds. In most cases, photoluminescent
radiation tends to be at longer wavelengths than
excitation radiation. Chemiluminescence is based
on an excited species formed by a chemical
reaction.
2
Features of Luminescent Methods High
sensitivity detection limits 1-3 orders of
magnitude better than in absorption
spectrometry. Large linear concentration range
(wider than absorption). Good selectivity. Much
less applicable than absorption methods.
3
Types of Fluoresence/phosphorescence Resonance
radiation (or fluorescence) absorbed
radiation is reemitted without alteration. More
often, molecular fluorescence (phosphorescence)
occurs as bands centered at wavelengths longer
than resonance line. This shift to longer
wavelengths is Stokes shift.
4
Electronic States Singlet State electron spins
paired, no splitting of energy level. May be
ground or excited state. Doublet State free
radical (due to odd electron). Triplet State
one electron excited to higher energy state,
spin becomes unpaired (parallel).
5
Energy-level diagram for typical photoluminescent
system
Source Skoog, Holler, and Nieman, Principles of
Instrumental Analysis, 5th edition, Saunders
College Publishing.
6
Examples of Luminescence Measurements
Source I. M. Warner, G.Patoney, and M. P.
Thomas, Anal. Chem., 57, 463A-482A (1985)..
7
Qauntum Yield Quantum yield for a fluorescent
process is the ratio of the number of molecules
that fluoresce to the total number of excited
molecules.
8
Variables that Affect Fluorescence Temperature
increased temperature, decreased quantum
yield Solvent Viscosity lower viscosity, lower
quantum yield Fluorescence usually
pH-dependent Dissolved oxygen reduces emission
intensity Concentration Self-quenching due
to collisions of excited molecules.
Self-absorbance when fluorescence emission and
absorbance wavelengths overlap.
9
Source Skoog, Holler, and Nieman, Principles of
Instrumental Analysis, 5th edition, Saunders
College Publishing.
10
Sample Spectra Excitation (left), measure
luminescence at fixed wavelength while varying
excitation wavelength. Fluorescence (middle) and
phosphorescence (right), excitation is fixed and
record emission as function of wavelength.
Source Skoog, Holler, and Nieman, Principles of
Instrumental Analysis, 5th edition, Saunders
College Publishing.
11
Nearly all fluorometers (spectrofluorometers)
are double-beam systems.
Source Skoog, Holler, and Nieman, Principles of
Instrumental Analysis, 5th edition, Saunders
College Publishing.
12
Sample Excitation and Emission Spectra
Excitation
Emission
Source Skoog, Holler, and Nieman, Principles of
Instrumental Analysis, 5th edition, Saunders
College Publishing.